Transthyretin Amyloid Cardiomyopathy (ATTR-CM) Market Size
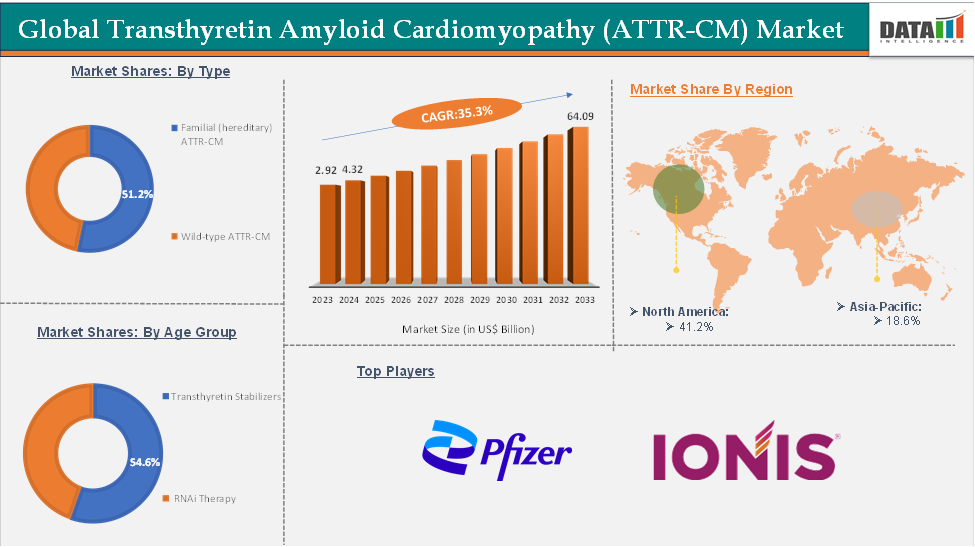
The global transthyretin amyloid cardiomyopathy (ATTR-CM) was valued at US$ 2.92 Billion n 2023. The global transthyretin amyloid cardiomyopathy (ATTR-CM) market size reached US$ 4.32 Billion in 2024 and is expected to reach US$ 64.09 Billion by 2033, growing at a CAGR of 35.3% during the forecast period 2025-2033.
Transthyretin Amyloid Cardiomyopathy (ATTR-CM) Market Overview
The transthyretin amyloid cardiomyopathy (ATTR-CM) market is witnessing substantial growth, primarily driven by increasing disease awareness, advancements in diagnostic technologies, and the growing availability of novel therapeutics.
Key opportunities in the market include ongoing clinical trials, rising investments in rare disease research, and the expansion of patient access programs, particularly in underserved regions.
North America currently dominates the ATTR-CM market due to early adoption of advanced therapies, robust healthcare infrastructure, and strong presence of key pharmaceutical players. However, the Asia-Pacific region is emerging as the fastest-growing market, supported by rising healthcare expenditure, improving diagnostic capabilities, and growing recognition of amyloidosis-related conditions among clinicians.
Transthyretin Amyloid Cardiomyopathy (ATTR-CM) Market – Executive Summary
Rising prevalence & aging population are significantly driving the transthyretin amyloid cardiomyopathy (ATTR-CM) market growth
The rising prevalence of Transthyretin Amyloid Cardiomyopathy (ATTR-CM) is a key driver of market growth, fueled by advancements in non-invasive diagnostics and improved patient outcomes. Innovations such as technetium pyrophosphate nuclear cardiac imaging now allow for accurate diagnosis without the need for cardiac biopsy, leading to earlier and broader identification of patients. In the U.S. alone, approximately 5,000 to 7,000 new cases are now diagnosed annually.
Studies indicating a prevalence of around 20% in heart failure patients with myocardial wall thickening over 14 mm highlight the growing clinical awareness and diagnostic precision. Together, these factors are driving demand for effective treatment options and expanding the ATTR-CM therapeutics market.
Restraint:
High cost of therapy hampering the growth of the transthyretin amyloid cardiomyopathy (ATTR-CM) market
The high cost of therapy remains a significant restraint for the Transthyretin Amyloid Cardiomyopathy (ATTR-CM) market. Current approved treatments, such as tafamidis (Vyndaqel/Vyndamax), can cost over $225,000 annually in markets like the U.S., posing a serious financial burden for patients and healthcare systems. Despite insurance coverage, out-of-pocket expenses can be substantial, limiting access, especially for underinsured or elderly patients on fixed incomes.
Moreover, in many low- and middle-income countries, such therapies are either unavailable or unaffordable, further widening the treatment gap. High costs also strain national healthcare budgets, making widespread reimbursement and adoption of advanced therapies challenging. As new therapies enter the market, pricing pressure and demand for cost-effective alternatives may intensify, but until then, affordability remains a major barrier to equitable access and market penetration.
Opportunity:
Expansion into emerging markets are expected to create a lucrative opportunity for the growth of the transthyretin amyloid cardiomyopathy (ATTR-CM) market
Expansion into emerging markets presents a significant opportunity for growth in the Transthyretin Amyloid Cardiomyopathy (ATTR-CM) market. Many developing countries in regions such as Latin America, Southeast Asia, and parts of the Middle East are experiencing a rapid increase in aging populations, which directly correlates with a higher incidence of ATTR-CM.
Historically, limited diagnostic infrastructure and low disease awareness have hindered detection and treatment in these areas. However, as healthcare systems in these regions modernize, there is increasing investment in rare disease diagnostics, better access to advanced imaging, and improved training for clinicians.
Moreover, pharmaceutical companies are beginning to target these markets with expanded clinical trials, partnerships, and affordable pricing models. By addressing unmet needs and broadening therapeutic availability, expansion into emerging markets not only improves global patient outcomes but also unlocks new revenue streams and drives sustained market growth.
For more details on this report – Request for Sample
Transthyretin Amyloid Cardiomyopathy (ATTR-CM) Market, Segment Analysis
The global transthyretin amyloid cardiomyopathy (ATTR-CM) market is segmented based on type, drug type, distribution channel, and region.
The transthyretin stabilizers in the drug type segment are expected to hold 54.6% of the market share in 2024 in the transthyretin amyloid cardiomyopathy (ATTR-CM) market
The transthyretin stabilizers segment is expected to dominate the market. This dominance is primarily due to the widespread adoption and clinical success of tafamidis (Vyndaqel/Vyndamax), the first FDA-approved drug for ATTR-CM. Its proven efficacy in reducing all-cause mortality and cardiovascular-related hospitalizations has positioned it as the standard of care.
For instance, in July 2023, BridgeBio Pharma announced positive Phase 3 results from its ATTRibute-CM trial evaluating acoramidis, a next-generation, orally-administered transthyretin stabilizer for the treatment of transthyretin amyloid cardiomyopathy (ATTR-CM). The study confirmed both the efficacy and safety of acoramidis, reinforcing its potential as a powerful therapeutic option. As a highly potent small molecule designed to stabilize transthyretin (TTR), acoramidis represents a significant advancement in ATTR-CM treatment. This development further strengthens the competitive landscape of transthyretin stabilizers and supports the continued market dominance of this therapy class.
Additionally, its oral administration route enhances patient compliance compared to more invasive treatments. Strong backing from clinical trials, increasing physician familiarity, and early diagnosis through advanced imaging have further driven the uptake of stabilizer therapies, reinforcing their leading position in the ATTR-CM market.
Transthyretin Amyloid Cardiomyopathy (ATTR-CM) Market, Geographical Analysis
North America is expected to dominate the global transthyretin amyloid cardiomyopathy (ATTR-CM) market with a 41.2% share in 2024
North America is expected to dominate the transthyretin amyloid cardiomyopathy (ATTR-CM) market, driven by strong clinical research activity, early adoption of advanced therapies, and robust regulatory support. For instance, in February 2024, Ionis Pharmaceuticals and AstraZeneca received Fast Track designation from the U.S. FDA for eplontersen, an investigational RNA-targeted therapy for ATTR-CM, reflecting the region's proactive stance in accelerating drug development for unmet medical needs.
Additionally, in May 2024, BridgeBio Pharma announced positive Phase 3 clinical results for acoramidis during the 2024 International Symposium of Amyloidosis, further underscoring North America’s leadership in ATTR-CM innovation. Coupled with high disease awareness, strong healthcare infrastructure, and favorable reimbursement environments, these advancements solidify North America’s position as the dominant regional market for ATTR-CM therapies.
Asia-Pacific is growing at the fastest pace in the transthyretin amyloid cardiomyopathy (ATTR-CM) market, holding 18.6% of the market share
Asia-Pacific is emerging as the fastest-growing region in the Transthyretin Amyloid Cardiomyopathy (ATTR-CM) market due to a combination of rising disease awareness, improving diagnostic capabilities, and increasing investments in rare disease treatment. Countries such as Japan, China, and South Korea are witnessing a growing elderly population, creating a larger at-risk patient pool.
Collaborations with local healthcare providers and favorable regulatory reforms aimed at expediting drug approvals are further accelerating the adoption of ATTR-CM therapies in the region. These factors collectively contribute to the region's rapid market expansion and position Asia-Pacific as the fastest-growing market for ATTR-CM treatment.
Transthyretin Amyloid Cardiomyopathy (ATTR-CM) Market Competitive Landscape
Top companies in the transthyretin amyloid cardiomyopathy (ATTR-CM) market include Pfizer Inc., Ionis Pharmaceuticals, Inc., among others.
Transthyretin Amyloid Cardiomyopathy (ATTR-CM) Market Key Developments
In March 2025, Alnylam Pharmaceuticals announced that the U.S. FDA approved a supplemental New Drug Application (sNDA) for AMVUTTRA (vutrisiran), expanding its use to include the treatment of cardiomyopathy of wild-type or hereditary transthyretin-mediated amyloidosis (ATTR-CM) in adults.
In June 2025, Alnylam Pharmaceuticals announced that the European Commission (EC) approved AMVUTTRA (vutrisiran) for the treatment of wild-type or hereditary transthyretin amyloidosis with cardiomyopathy (ATTR-CM) in adults.
In September 2022, Intellia Therapeutics and Regeneron Pharmaceuticals reported positive interim Phase 1 results for NTLA-2001, an investigational CRISPR/Cas9-based in vivo gene editing therapy for ATTR amyloidosis. The study included 12 adult patients with ATTR-CM and NYHA Class I–III heart failure, showing promise for single-dose, potentially curative treatment.
Transthyretin Amyloid Cardiomyopathy (ATTR-CM) Market Scope
Metrics | Details | |
CAGR | 35.3% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Type | Familial (hereditary) ATTR-CM, Wild-type ATTR-CM |
Drug Type | Transthyretin Stabilizers, RNAi Therapy, Others | |
Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
DMI Insights:
The global transthyretin amyloid cardiomyopathy (ATTR-CM) market, valued at US$ 2.92 Billion in 2023, grew to US$ 4.32 Billion in 2024 and is projected to reach US$ 64.09 Billion by 2033, expanding at a robust CAGR of 35.3% from 2025 to 2033. This rapid growth is driven by advancements in RNAi therapies, transthyretin stabilizers, and non-invasive diagnostic tools such as technetium pyrophosphate imaging. North America currently leads the market, fueled by early adoption of novel treatments, a well-established healthcare infrastructure, and the presence of major pharmaceutical players.
The Asia-Pacific region is witnessing the fastest growth, supported by increasing healthcare spending, improved access to diagnostics, and rising awareness of amyloidosis-related cardiac conditions. Although high treatment costs remain a barrier, ongoing R&D, favorable regulatory designations, and expanding clinical trial activity are bolstering the pipeline and accelerating market expansion globally.
The global transthyretin amyloid cardiomyopathy (ATTR-CM) market report delivers a detailed analysis with 60+ key tables, more than 55+ visually impactful figures, and 178 pages of expert insights, providing a complete view of the market landscape.

