NASH/MASH Treatment Market Size
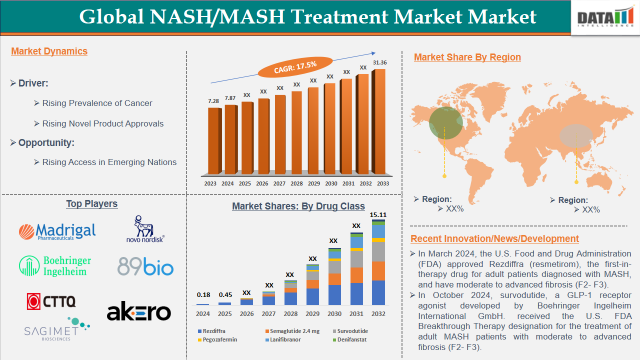
The Global NASH/MASH Treatment Market reached US$ 7.87 billion in 2024 and is expected to reach US$ 31.76 billion by 2033, growing at a CAGR of 17.7% during the forecast period 2025-2033. The approved drugs market value is reported at US$ 178.31 million in 2024 and is expected to reach US$ 16.82 billion in 2033 growing at a CAGR of 57.05%.
Metabolic Dysfunction-Associated Steatohepatitis (MASH) formerly known as Nonalcoholic Steatohepatitis (MASH) is a disease caused by a build-up of fat in the liver, not caused by alcohol consumption. As a result of fat deposition, the liver becomes inflamed (hepatitis). The inflammation and liver damage from MASH can cause fibrosis and can lead to cirrhosis, where the liver is scarred and significantly damaged, often permanently.
The risk factors for mash include several underlying conditions such as obesity, type 2 diabetes & insulin resistance, metabolic syndrome, and abnormal levels of fat in the blood. The common symptoms include abdominal discomfort and pain, fatigue, loss of appetite, weight loss, swelling in legs and jaundice, etc.
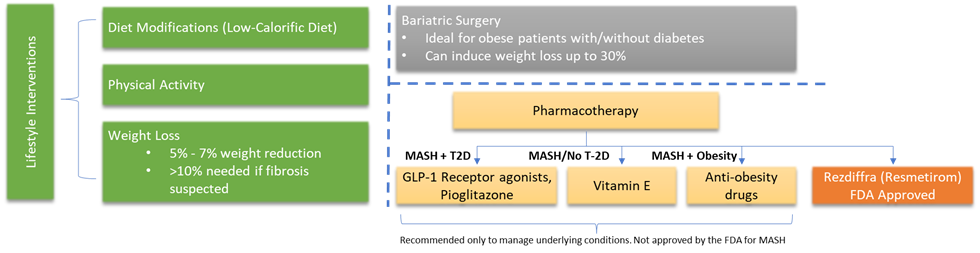
The treatment options for MASH include lifestyle interventions, bariatric surgery, and pharmacotherapy. Lifestyle interventions include diet modifications, weight reduction, improving physical exercise, etc. Bariatric surgery is indicated in patients with obesity/without diabetes and in whom the BMI is ≥ 35 kg/m2. The pharmacotherapy intervention is based on the underlying condition and is usually prescribed along with lifestyle interventions. If the patient is diagnosed with MASH and has type 2 diabetes, the choice of drugs includes GLP-1 Receptor agonists with lifestyle interventions. If the patient is diagnosed with MASH and have obesity the choice of pharmacotherapy includes anti-obesity drugs such as statins along with lifestyle interventions. If the patient is diagnosed with MASH and has no diabetes, vitamin E is generally prescribed along with lifestyle interventions. Recently a novel first-in-therapy drug Resmetirom sold under the brand name Rezdiffra was approved by the U.S. FDA for the treatment of adult MASH patients with moderate to advanced liver fibrosis.
Below is the common treatment algorithm for diagnosed MASH patients.
Executive Summary
For more details on this report – Request for Sample
Market Dynamics: Drivers & Restraints
Rising Prevalence of Underlying Conditions
The rising prevalence of underlying conditions such as obesity, type 2 diabetes, and other metabolic syndrome is a key factor driving the growth of the MASH (Non-Alcoholic Steatohepatitis/Metabolic Dysfunction-Associated Steatohepatitis) market. These conditions contribute significantly to the development of liver diseases, particularly MASH, which are increasingly recognized as public health concerns globally.
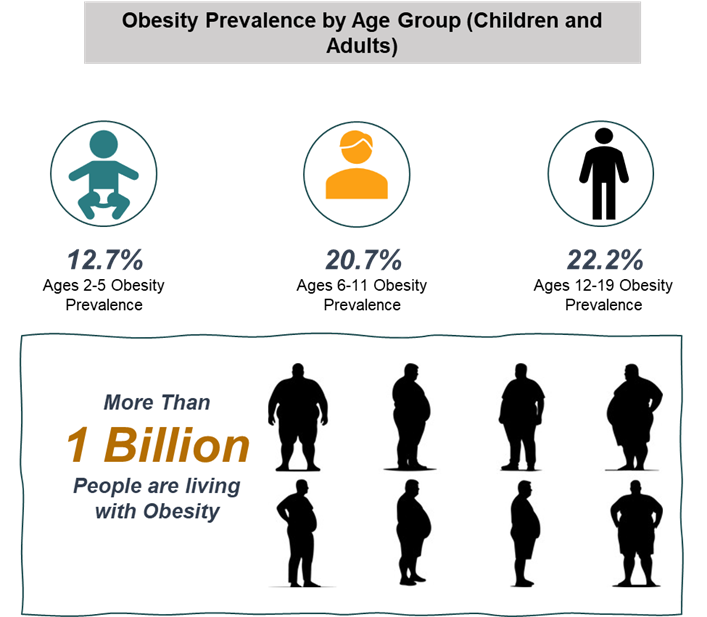
For instance, according to the World Obesity Federation estimates, more than 1 billion people globally are living with obesity, 880 million are adults and 159 million are children and adolescents aged 5-19 years. Obesity is a major risk factor for both MASH and MASH. The global obesity rate has surged over the past few decades, contributing directly to an increase in liver-related diseases.
In obese individuals, excess fat accumulation in the liver (known as NAFLD, Non-Alcoholic Fatty Liver Disease) can progress to more severe stages such as MASH. About 20-30% of people with NAFLD will eventually develop MASH, increasing the demand for effective treatments.
Type 2 diabetes is another major risk factor for MASH. The global prevalence of type 2 diabetes is increasing at an alarming rate, with the International Diabetes Federation estimating that by 2045, 1 in 8 adults, approximately 783 million, will be living with diabetes, an increase of 46%. Over 90% of people with diabetes have type 2 diabetes.
Type 2 diabetes leads to insulin resistance, a metabolic dysfunction that is strongly linked to fat accumulation in the liver (a hallmark of MASH). Additionally, the fatty liver associated with diabetes can exacerbate insulin resistance, creating a vicious cycle.
The growing prevalence of these underlying conditions has prompted pharmaceutical companies to accelerate the development of novel therapies for MASH. Currently, several treatments are in late-stage clinical trials, targeting different disease mechanisms such as fibrosis, liver inflammation, and insulin resistance.
Patient Compliance Issues May Restrain the Market Growth
Patient compliance issues are a significant factor hampering the growth of the market. Even though there is growing awareness about these diseases and the importance of early diagnosis, treatment adherence remains a challenge. Poor compliance with prescribed therapies, lifestyle changes, and long-term management plans can limit the effectiveness of treatments, prolong disease progression, and hinder the overall growth of the market.
MASH often requires long-term management, including consistent medications, regular check-ups, and significant lifestyle changes such as weight loss, exercise, and dietary modifications. However, patients may struggle with these long-term commitments, especially when they don't experience immediate, noticeable improvements.
Some medications used to treat MASH, particularly those in clinical trials or early-stage approval, come with side effects that can make long-term adherence difficult. Side effects such as gastrointestinal discomfort, fatigue, or liver-related issues can discourage patients from sticking to their treatment regimen.
Market Segment Analysis
The global NASH/MASH Treatment market is segmented based on drug, stage, age group, gender, and region.
Stage 2-3 in the stage segment is dominating the market.
The stage 2-3 segment in the NASH/MASH treatment market was valued at US$ 3,237.06 Million in 2024 and is estimated to reach US$ 19,391.01 Million by 2032, growing at a CAGR of 27.2% during the forecast period from 2025-2032.
In MASH fibrosis stage 2, the liver has undergone inflammation and damage, leading to some degree of scarring. Despite this scarring, the liver is likely still functioning effectively, meaning it can carry out its essential roles. Importantly, much of the damage at this stage can be repaired through lifestyle modifications such as adopting a healthier diet, increasing physical activity, and achieving weight loss. This stage presents a crucial opportunity for intervention to halt further progression of liver disease.
Resmetirom has become the first liver-specific medication to receive conditional approval from the FDA in the United States for treating patients with non-cirrhotic metabolic dysfunction-associated steatohepatitis (MASH), specifically those with fibrosis stages 2 or 3. The FDA label does not mandate the use of liver biopsy.
In MASH fibrosis stage 3, the liver has developed substantial scarring. At this advanced stage, it is crucial to prevent further damage and scarring to avoid worsening the condition. Although significant injury has occurred, there remains a possibility for some repair of the liver through lifestyle modifications and medical interventions. Taking early action is vital for effectively managing the disease and reducing the risk of progressing to more severe liver complications, such as cirrhosis.
In these stages, patients endure moderate to severe liver scarring resulting from ongoing inflammation and damage linked to MASH. This statistic underscores a significant public health issue, as it highlights a large population at increased risk for serious liver complications, including cirrhosis and liver failure.
Market Geographical Share
North America dominated the NASH/MASH Treatment market.
The North America region has the highest share of 47% in 2024 in the NASH/MASH treatment market. The region’s dominance is attributable to higher prevalence, diagnosis, and treatment rates combined with the availability of the approved drug Resmetirom.
The rising prevalence of underlying metabolic diseases such as obesity, type 2 diabetes, and hypertension in North America is the major driver for the market growth.
For instance, according to the Centers for Disease Control and Prevention (CDC), in the United States, the prevalence of obesity in adults was 40.3%, with no significant differences between men and women. More than 38 million Americans have diabetes (about 1 in 10), and about 90% to 95% of them have type 2 diabetes. Nearly 1 out of 2 adults in the United States has hypertension (116 million). This prevalence is expected to rise in the future, which will result in a significant rise in MASH cases. As per DataM estimates, the total prevalent cases of MASH in the U.S. were approximately 17.50 million in 2024, which is expected to reach 23.33 million by 2030.
The United States is a global hub for biopharmaceutical and biotech innovations focused on liver diseases, including MASH. Many pharmaceutical companies, such as Madrigal Pharmaceuticals, Inc., and Novo Nordisk, have made significant initiatives in developing therapies for MASH.
The rising prevalence combined with the anticipated market approvals in the forecast period, is expected to significantly drive the North America NASH/MASH treatment market.
Major Global Players
The major player in the NASH/MASH Treatment market is Madrigal Pharmaceuticals. The emerging players include Novo Nordisk A/S, Boehringer Ingelheim International GmbH., 89bio, Inc., Inventiva., CHIA TAI TIANQING PHARMACEUTICAL GROUP CO., LTD, Sagimet Biosciences., and Akero Therapeutics, Inc. among others.
Key Developments
- In March 2024, the U.S. Food and Drug Administration (FDA) approved Rezdiffra (resmetirom), the first-in-therapy drug for adult patients diagnosed with MASH, and have moderate to advanced fibrosis (F2- F3). Rezdiffra developed by Madrigal Pharmaceuticals, Inc. is a once-daily, oral THR-β agonist that has proven its safety and efficacy in Phase 3 MAESTRO-NASH trial.
- In October 2024, survodutide, a GLP-1 receptor agonist developed by Boehringer Ingelheim International GmbH. received the U.S. FDA Breakthrough Therapy designation for the treatment of adult MASH patients with moderate to advanced fibrosis (F2- F3).
| Metrics | Details | |
| CAGR | 17.7% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Mn) | |
| Segments Covered | Drug | Resmetirom (Rezdiffra), Lanifibranor, Semaglutide, Survodutide, Pegozafermin, Efruxifermin, Denifanstat, and Others |
| Stage | Stage 0-1, Stage 2-3, and Stage 4 | |
| Age-Group | Pediatrics, Adults, and Geriatrics | |
| Gender | Male, and Female | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials, and product pipelines, and forecasts upcoming pharmaceutical advancements.
- Type Performance & Market Positioning: Analyzes product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: Covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyzes competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient Type delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global melanoma therapeutics market report would provide approximately 54 tables, 46 figures, and 195 pages.
Target Audience 2023
- Manufacturers: Pharmaceutical, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.

