Global Lateral Flow Assays Market: Industry Outlook
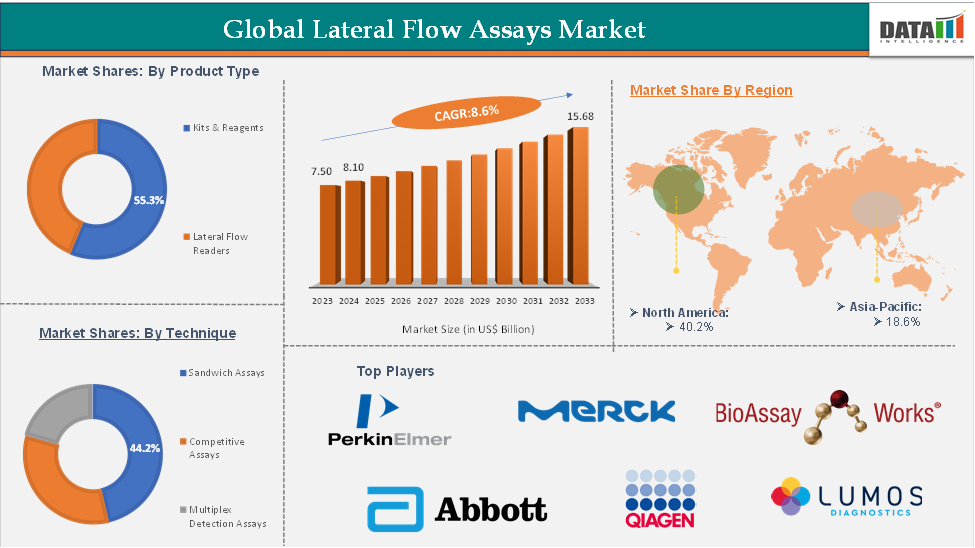
The global lateral flow assays market reached US$ 6.92 billion in 2023, with a rise of US$ 7.50 billion in 2024, and is expected to reach US$ 15.68 billion by 2033, growing at a CAGR of 8.6% during the forecast period 2025-2033.
Lateral flow assays (LFAs) are seeing increased adoption as demand grows for faster, more accessible diagnostic tools. One major driver of this growth is the increased prevalence of infectious diseases and chronic conditions. As health systems face mounting pressure to identify and manage conditions early, LFAs offer a reliable and rapid testing method that supports timely clinical decisions in both acute and routine care.
Another key factor is the growing adoption of at-home and self-testing kits. People are increasingly taking a proactive role in managing their health, and LFAs provide a convenient way to test for a range of conditions without visiting a clinic. This shift is not only improving access but also helping to reduce strain on laboratory services.
At the same time, there's growing interest in the use of LFAs in emerging and low-resource settings, where access to centralized labs may be limited. Their portability, ease of use, and minimal infrastructure requirements make them well-suited for community health programs and remote care delivery.
In addition, customization and private label manufacturing are opening new possibilities for more targeted solutions. Companies are tailoring LFAs to address specific conditions, use cases, and branding needs, allowing for greater flexibility and scalability in development and deployment. Together, these trends are reinforcing the role of LFAs as a vital tool in delivering fast, practical, and accessible diagnostics across a broad range of healthcare settings.
Executive Summary
Dynamics: Drivers & Restraints
Driver: Increased Prevalence of Infectious Diseases and Chronic Conditions
The escalating global burden of infectious diseases and chronic health conditions is a significant driver of growth in the lateral flow assays (LFA) market. LFAs are increasingly utilized at the point of care to diagnose a wide array of infections caused by viruses (such as HIV, hepatitis, and SARS-CoV-2), bacteria (like streptococcus and E. coli), and parasites (including malaria). Their rapid, user-friendly, and cost-effective nature makes them particularly valuable in managing the growing number of health threats worldwide.
According to the World Health Organization (WHO), an estimated 254 million people were living with chronic HBV infection in 2022, with 1.2 million new infections occurring annually. The burden is particularly high in the WHO Western Pacific and African Regions, where 97 million and 65 million individuals are chronically infected, respectively. Other regions also report substantial numbers: 61 million in South-East Asia, 15 million in the Eastern Mediterranean, 11 million in Europe, and 5 million in the Americas.
The rise in infectious diseases has underscored the need for swift and accessible diagnostic solutions. LFAs have proven instrumental in providing immediate results, enabling timely interventions and reducing the spread of infections. For instance, during the COVID-19 pandemic, LFAs played a crucial role in mass testing efforts, facilitating early detection and containment measures.
Beyond infectious disease detection, LFAs are expanding into chronic disease management, environmental monitoring, and food safety applications. They are now employed in detecting cancer markers, monitoring cardiac biomarkers, and assessing environmental toxins and foodborne pathogens. As the prevalence of both infectious and chronic diseases continues to rise globally, the demand for rapid, reliable, and accessible diagnostic tools like LFAs is expected to grow, further propelling the expansion of the LFA market.
Restraint: Lower Sensitivity Compared to Lab-Based Methods
Despite their convenience and speed, one of the major challenges facing LFAs is their relatively lower sensitivity when compared to centralized, lab-based methods such as PCR or ELISA. This limitation can lead to false negatives, especially in the early stages of infection when biomarker levels are low. As a result, clinical decisions based solely on LFA results may require confirmatory testing. This sensitivity gap can restrict the broader adoption of LFAs in certain high-stakes or highly regulated clinical settings, potentially slowing market growth in those segments.
For more details on this report, Request for Sample
Segmentation Analysis
The global lateral flow assays market is segmented based on product, technique, application, end-user, and region.
Product:
The kits and reagents segment is estimated to have 55.3% of the lateral flow assays market share.
The kits and reagents segment is projected to maintain a dominant position in the lateral flow assays (LFA) market, primarily due to their recurring demand and integral role in diagnostic testing across various applications. These kits offer a comprehensive solution, typically containing pre-measured reagents, test strips, and buffers, which simplifies the testing process and ensures consistency and reliability. Their user-friendly design eliminates the need for specialized equipment or extensive training, making them ideal for point-of-care settings, including hospitals, clinics, and home testing environments.
The growth of this segment is further fueled by several factors. The increasing prevalence of infectious diseases and chronic conditions has heightened the demand for rapid and accessible diagnostic tools. Additionally, the rising adoption of at-home and self-testing kits reflects a shift towards more patient-centric healthcare models, where individuals seek greater control over their health management.
Technological advancements, such as the integration of nanotechnology and multiplexing capabilities, have enhanced the sensitivity and specificity of LFAs, broadening their applicability and reliability. Moreover, innovations in assay chemistry and reagent stabilization have improved the shelf-life and performance of these kits, contributing to their widespread use and sustained market growth.
Geographical Share Analysis
The North America lateral flow assays market was valued at 40.2% market share in 2024
North America is projected to maintain its leadership in the lateral flow assays (LFA) market in the coming years, driven by several key factors. The region boasts a well-established healthcare infrastructure, characterized by advanced medical facilities, a high density of diagnostic centers, and widespread adoption of point-of-care testing technologies. This robust infrastructure facilitates the efficient deployment and utilization of LFAs across various healthcare settings, including hospitals, clinics, and home care environments.
Additionally, the strong regulatory frameworks in countries like the United States, exemplified by agencies such as the FDA, ensure the safety, efficacy, and rapid approval of diagnostic tools, fostering a favorable environment for the proliferation of LFAs.
The COVID-19 pandemic significantly accelerated the adoption of LFAs, as the need for rapid, accessible, and widespread testing became paramount. In response, ongoing investments in healthcare innovation have been directed towards enhancing the capabilities of LFAs, including improvements in sensitivity, multiplexing, and integration with digital health platforms. For instance, in July 2025, VolitionRx Limited developed a lateral flow device capable of quantifying nucleosomes in whole venous blood within minutes. In a blinded study involving 25 patients from intensive care and emergency departments, the device's results strongly correlated with those of Volition's established Nu. Q nucleosome assay. This advancement enables rapid, bedside detection of immune disruptions, such as those occurring in sepsis, without the need for laboratory testing.
These advancements are further propelling the adoption of LFAs in diverse applications, ranging from infectious disease detection to chronic disease monitoring and personalized medicine.
Furthermore, the increasing prevalence of chronic and infectious diseases in the region has amplified the demand for efficient diagnostic tools. Together, these factors position North America to continue its dominance in the LFA market, with sustained growth anticipated in the foreseeable future.
Major Players
The major players in the lateral flow assays market include Getinge, STERIS, Skytron, LLC, Mizuho OSI, Merivaara Corp., Hill-Rom Holdings, Inc., Medifa, Boyd Industries, and SCHMITZ medical GmbH, among others.
Market Scope
Metrics | Details | |
CAGR | 8.6% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Product | Kits & Reagents, Lateral Flow Readers |
Technique | Sandwich Assays, Competitive Assays, Multiplex Detection Assays | |
| Application | Drug Development & Quality Testing, Food Safety & Environmental Safety, Pregnancy & Fertility Testing, Cardiac Markers, Infectious Diseases Testing, Drugs of Abuse Testing, Clinical Testing, Cholesterol/Lipid Testing, Other Clinical Tests |
| End-User | Pharmaceutical and Biotechnology Companies, Hospitals and Clinics, Diagnostic Laboratories, Home Care, Others |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global lateral flow assays market report delivers a detailed analysis with 73 key tables, more than 76 visually impactful figures, and 195 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more hospital supplies-related reports, please click here
