Disease Overview:
Celiac disease is a chronic autoimmune disorder that damages the small intestine. It develops in individuals with a genetic predisposition, where consuming gluten triggers an immune response that harms the lining of the small intestine. This condition arises from an intolerance to gluten, a protein found in wheat, barley, and rye.
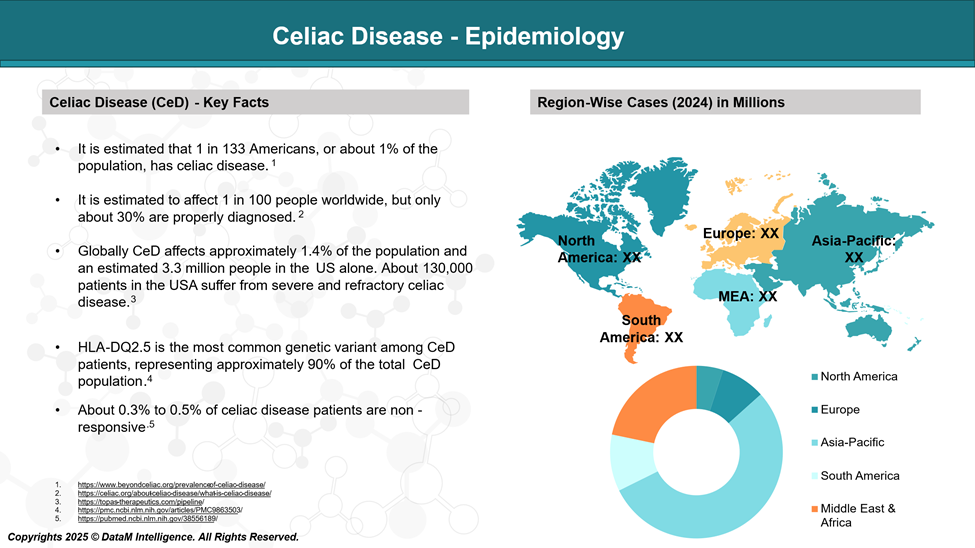
Epidemiology Analysis (Current & Forecast)
Celiac disease is a relatively common condition, affecting approximately 1% of the global population. However, these estimates only account for diagnosed cases, as a significant number of people with the disease may go undetected and remain unaware of their condition.
Approved Drugs - Sales & Forecast
Currently, there is no cure for celiac disease. The only effective and widely accepted treatment is:
Strict, lifelong gluten-free diet
This involves entirely eliminating foods that contain wheat, barley, and rye from the diet. Cutting out gluten allows the small intestine to recover and helps prevent additional harm.Supportive Treatments
While diet is the cornerstone, additional management strategies include:
Nutritional supplements
Iron (for anemia)
Calcium and vitamin D (for bone health)
B12, folate, and other vitamins if deficiencies are present
Treatment for symptoms/complications
Anti-diarrheal or constipation medications
Steroids (in rare, severe, or refractory cases)
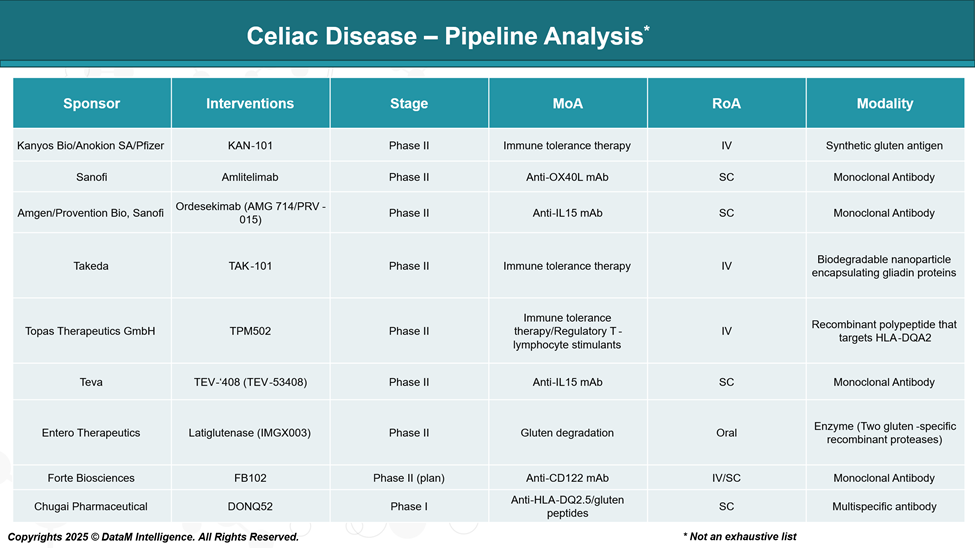
Pipeline Analysis and Expected Approval Timelines
Researchers are working on new therapies for celiac disease that may support or potentially serve as alternatives to the gluten-free diet. These new options work in different ways, such as calming the immune system, breaking down gluten in the body, or protecting the gut lining from damage.
Competitive Landscape and Market Positioning
Currently, there are no FDA-approved pharmacological therapies to treat celiac disease. The therapeutic landscape is dominated by the gluten-free diet, which remains the only standard of care. However, the unmet clinical need, particularly for individuals with non-responsive or severe forms of the disease, has driven the development of several novel agents now advancing through clinical trials.
Emerging therapies are targeting different mechanisms involved in the disease, including immune tolerance induction, cytokine inhibition, gluten detoxification, and modulation of T-cell responses. These therapies are currently in Phase I or II trials, indicating a highly active early-stage pipeline.
Sponsor | Asset | Mechanism of Action | Differentiating Feature | Target Patient Segment | Potential Use |
Kanyos Bio / Anokion SA / Pfizer | KAN-101 | Immune tolerance via T-cell reprogramming | Antigen-specific immune retraining | All celiac patients, early to moderate disease | Long-term therapy or induction |
Sanofi | Amlitelimab | Anti-OX40L mAb (T-cell co-stimulation blockade) | Novel OX40L blockade pathway | Non-responsive or refractory celiac | Adjunct to GFD or rescue therapy |
Amgen / Provention Bio / Sanofi | Ordesekimab (AMG 714 / PRV-015) | Anti-IL-15 mAb | Focused on IL-15-driven inflammation | Refractory celiac disease | Monotherapy for inflammation control |
Takeda | TAK-101 | Nanoparticle-delivered gliadin for immune tolerance | Precision targeting via nanoparticle tech | Mild-to-moderate celiac | Immune retraining/maintenance |
Topas Therapeutics | TPM502 | Antigen-coupled nanoparticles stimulate Tregs | Treg-directed tolerance induction | New diagnosis or early intervention | Preventive or early-stage therapy |
Teva | TEV-‘408 (TEV-53408) | Anti-IL-15 mAb | Competing IL-15 pathway with alternative mAb | Refractory or severe cases | Symptom reduction or disease control |
Entero Therapeutics | Latiglutenase (IMGX003) | Oral enzyme to degrade gluten peptides | Direct gluten detoxification in GI tract | Accidental exposure, dietary lapses | Adjunct to GFD |
Forte Biosciences | FB102 | Anti-CD122 mAb (targets IL-2/IL-15 signaling) | Dual IL-2/IL-15 axis disruption | Moderate-to-severe inflammation | Targeted immune suppression |
Chugai | DONQ52 | Anti-HLA-DQ2.5/gluten complex blocking | First-in-class HLA-gluten interface target | HLA-DQ2.5-positive patients | Prevention of immune activation |
Market Positioning Insights
KAN-101, TAK-101, and TPM502 are leading in the space of immune tolerance therapies, aiming for disease-modifying potential rather than symptomatic relief.
Anti-cytokine therapies like Ordesekimab and TEV-‘408 are positioned as immunomodulators, particularly useful for refractory celiac disease.
Latiglutenase offers a unique niche as an oral enzyme therapy, likely to be positioned as an adjunct to the gluten-free diet for protection against accidental gluten exposure.
Early-stage candidates like DONQ52 represent first-in-class approaches to block the core autoimmune trigger at the HLA-peptide interface, setting them apart mechanistically.
Key Companies:

Target Opportunity Profile (TOP)
The Target Opportunity Profile (TOP) for emerging therapies in celiac disease summarizes the ideal characteristics a new drug candidate should meet to successfully enter and compete in the market.
Target Opportunity Profile: Celiac Disease Drug Development
Attribute | Target Profile for Market Entry & Success |
Safety | - Excellent safety and tolerability profile |
Efficacy | - Significant reduction in mucosal damage (villous atrophy) on biopsy |
Mechanism of Action (MoA) | - Novel, disease-relevant MoA (e.g., immune tolerance induction, cytokine pathway blockade, gluten detoxification) |
Route of Administration (RoA) | - Oral preferred for adherence and patient convenience |
Innovation Level | - First-in-class or best-in-class status |
Therapeutic Modality | - Biologics (e.g., mAbs), engineered enzymes, peptides, or nanoparticle delivery systems |
Patient Subgroup Targeting | - Flexibility to treat: |
Commercial Viability | - Address large underserved market (up to 80% undiagnosed) |
Why Buy Our Pharma Competitive Intelligence Report?
Our Pharma Competitive Intelligence Report is designed to give you a strategic advantage by providing deep insights into the pharmaceutical landscape. Here’s how it benefits you and your business: