Travelers Vaccines Market Size
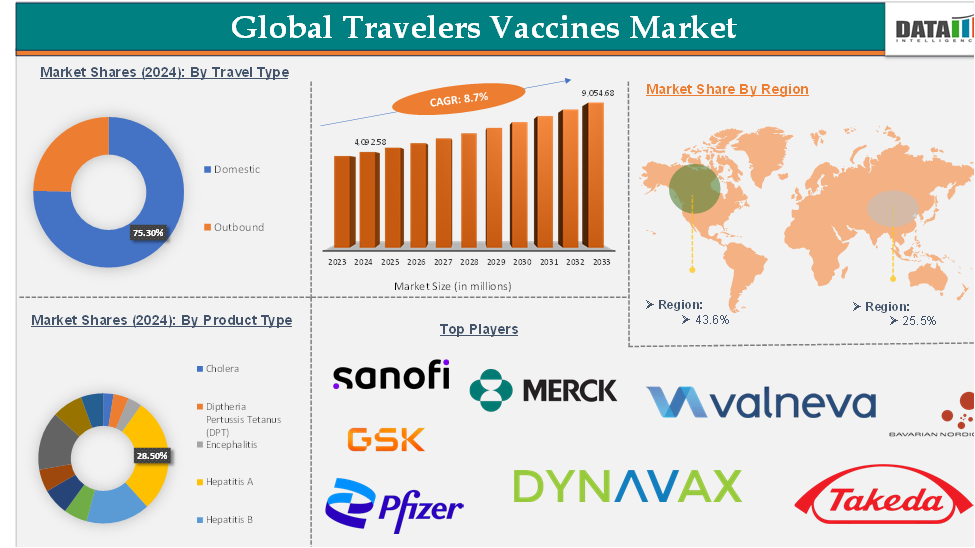
Travelers Vaccines Market reached US$ 4,092.58 million in 2024 and is expected to reach US$ 9,054.68 million by 2033, growing at a CAGR of 8.7 % during the forecast period of 2025-2033.
The global traveler's vaccines market encompasses the development, manufacturing, distribution, and administration of vaccines tailored to protect travelers from infectious diseases commonly found or endemic in specific regions worldwide. Also known as travel immunizations, these vaccines are recommended or mandated for individuals traveling internationally to ensure their safety and to help prevent the transmission of diseases across national borders.
Key drivers include the prevalence of vaccine-preventable diseases in travel destinations, expanding tourism infrastructure, and public-private partnerships improving vaccine accessibility and affordability. Opportunities are emerging from the adoption of digital health technologies, the expansion of vaccination centers, and tailored vaccination strategies for specific traveler profiles.
The scope of the market extends across various vaccine types (e.g., hepatitis A, typhoid, yellow fever), travel types (domestic and outbound), and distribution channels, with demand particularly strong for vaccines targeting diseases prevalent in popular tourist destinations.
Industry outlook remains positive, fueled by trends such as the increasing frequency of international travel, the emergence of infectious disease threats, government regulations mandating vaccination, and the growing popularity of combination vaccines that offer protection against multiple diseases.
Travelers Vaccines Market - Executive Summary
For more details on this report, Request for Sample
Market Scope
| Metrics | Details | |
| CAGR | 8.7% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Mn) | |
| Segments Covered | Product Type | Cholera, Diphtheria Pertussis Tetanus (DPT), Encephalitis, Hepatitis A, Hepatitis B, Meningococcal, Influenza, Rabies, Typhoid, Yellow Fever, Others |
| Travel Type | Domestic, Outbound | |
| End-User | Hospitals, Travel Clinics, Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
Travelers Vaccines Market Dynamics
Rising International Travel and Tourism
The surge in international travel and tourism is a primary driver fueling the growth of the global travelers' vaccines market. As global mobility increases, more individuals are traveling to regions where they may be exposed to infectious diseases not prevalent in their home countries. This heightened movement-whether for business, leisure, adventure, or visiting friends and relatives-significantly raises the risk of contracting and spreading vaccine-preventable diseases, thereby boosting demand for travel vaccines.
The direct correlation between rising international travel and increased demand for travel vaccines is evident in the market's robust growth projections. It increases the exposure of travelers to infectious diseases, prompts regulatory vaccination requirements, and heightens health awareness, all of which contribute to sustained and growing demand for travel vaccines worldwide.
Additionally, key players in the industry initiatives that would drive this market growth. For instance, in March 2024, Umrah and Hajj tour operators, along with UAE residents, are seeking further clarification after the Ministry of Health and Prevention (MoHAP) announced that influenza vaccination is now mandatory for all pilgrims traveling to Saudi Arabia. Travelers must present valid influenza vaccination cards as part of the health requirements for entry.
Also, in February 2024, UK retail pharmacies introduced a comprehensive new service to simplify international travel preparation. This service offers travelers personalized counseling based on their travel itinerary, ensuring they receive all required and recommended vaccines for their destination. Available vaccines include yellow fever, Japanese encephalitis, typhoid, and cholera, among others. All these factors demand the global traveler's vaccine market.
Stringent Regulatory Approvals
Stringent regulatory approvals represent a major restraint in the global traveler's vaccines market due to the complex, lengthy, and resource-intensive processes required to bring a new vaccine to market. These regulatory frameworks are designed to ensure that all vaccines meet the highest standards of safety, efficacy, and quality, but they also introduce several challenges for vaccine developers and manufacturers.
Vaccine development and approval involve multiple stages, including preclinical research, three phases of clinical trials, and extensive review of manufacturing processes and facilities. In the United States, for example, developers must submit an Investigational New Drug (IND) application before clinical trials, followed by phased human trials to assess safety, immunogenicity, and efficacy.
After successful trials, a Biological License Application (BLA) is submitted to the FDA, which undergoes a rigorous review that can take from six months (priority review) to a year (standard review). Similarly, in Europe, developers must submit comprehensive data to the European Medicines Agency (EMA) for scientific evaluation, which typically takes up to 210 days under standard timelines. Thus, the above factors could be limiting the global traveler's vaccine market's potential growth.
Travelers Vaccines Market Segment Analysis
The global traveler's vaccines market is segmented based on product type, travel type, end-user, and region.
Product Type:
The hepatitis A segment was valued at US$ 1,138.46 million in 2024 and is estimated to reach US$ 2,436.14 million by 2032, growing at a CAGR of 7.8% during the forecast period from 2025-2033
The Hepatitis A segment is a key component of the global traveler's vaccines market, driven by the need to protect international travelers from the Hepatitis A virus (HAV) infection. Hepatitis A is a highly contagious liver infection transmitted primarily through contaminated food and water, a risk that is especially high in regions with poor sanitation and limited access to clean water.
The demand for Hepatitis A vaccines is driven by both the high prevalence of the disease in popular travel destinations and the effectiveness of the vaccine itself. A single-dose schedule provides substantial short-term protection, while a two-dose series offers long-lasting immunity-often for decades.
Hepatitis A vaccination is strongly recommended for travelers visiting endemic areas, including parts of Africa, Asia, Central and South America, and Eastern Europe. The vaccine is often included in pre-travel health consultations and is one of the most commonly administered travel vaccines.
For instance, in January 2024, India's launch of its first indigenously developed Hepatitis A vaccine marks a significant milestone in both the national and global travelers' vaccines market. By producing a Hepatitis A vaccine locally, India can offer this crucial immunization at a lower cost compared to imported alternatives. This makes the vaccine more accessible not only to Indian travelers but also to neighboring countries and regions where cost has been a major barrier to vaccination. These factors have solidified the segment's position in the global traveler's vaccines market.
Travelers Vaccines Market Geographical Share
The North America travelers' vaccines market was valued at US$ 1,754.81 million in 2024 and is estimated to reach US$ 3,817.62 million by 2033, growing at a CAGR of 8.1% during the forecast period from 2025-2033
North America, particularly the United States and Canada, sees millions of international travelers each year for business, tourism, education, and family visits. This high volume of cross-border movement increases the demand for travel-related vaccinations to protect against region-specific infectious diseases.
The region benefits from advanced healthcare systems, widespread availability of travel clinics, and retail pharmacy vaccination services. This infrastructure ensures easy access to vaccines and pre-travel health consultations, making it convenient for travelers to get immunized. Travelers in North America generally have high awareness of travel health risks and the importance of immunization. Public health campaigns, CDC travel advisories, and educational resources contribute to informed decision-making and higher vaccine uptake rates.
In the U.S. and Canada, health authorities, such as the CDC and Health Canada, regularly update and enforce guidelines for travel vaccinations. Certain destinations require proof of vaccination (e.g., yellow fever), and these regulatory requirements drive compliance among travelers. The market offers a wide range of vaccines, including combination vaccines (e.g., Hepatitis A & B), single-dose options, and vaccines for emerging diseases. Innovation in vaccine technology and delivery methods (such as needle-free injectors) enhances convenience and adoption.
For instance, in March 2025, Bavarian Nordic introduced VIMKUNYA (Chikungunya Vaccine, Recombinant) to the U.S. market, the first virus-like particle (VLP), single-dose vaccine designed to protect against chikungunya for individuals aged 12 and older. With this launch, VIMKUNYA becomes a significant addition to the global travel health landscape, offering essential protection for travelers heading to regions where the chikungunya virus is endemic. Thus, the above factors are consolidating the region's position as a dominant force in the global travelers' vaccines market.
The Asia-Pacific travelers' vaccines market was valued at US$ 1,067.91 million in 2024 and is estimated to reach US$ 2,677.58 million by 2033, growing at a CAGR of 9.5% during the forecast period from 2025-2033
The Asia-Pacific traveler's vaccines market is experiencing strong growth, propelled by increasing international (outbound) and domestic travel, rising awareness of travel-related infectious diseases, and evolving public health policies.
The market covers a broad range of vaccines, including those for cholera, DPT (diphtheria, pertussis, tetanus), encephalitis, hepatitis A and B, meningococcal disease, influenza, rabies, typhoid, yellow fever, and others. These vaccines are administered through various modalities such as chemotherapy, hormone therapy, targeted therapy, immunotherapy, radiation, surgery, and other supportive measures, although vaccination is the primary preventive approach.
Japan plays a significant role in the Asia-Pacific travelers' vaccine market. The country’s robust healthcare system, strong regulatory environment, and high levels of public health awareness contribute to a large and growing market for both domestic and outbound traveler vaccinations. Japan’s focus on disease prevention, especially for international travelers and during major events, further accelerates market growth.
Travelers Vaccines Market – Global Players
The major global players in the traveler's vaccines market include GSK plc, Merck & Co., Inc., Bavarian Nordic Inc., Pfizer Inc., Sanofi, Dynavax Technologies, Valneva SE, and Takeda Pharmaceutical Company Limited, among others.
Travelers Vaccines Market – Key Developments
- In April 2025, Valneva SE, a specialty vaccine company, announced that the European Commission granted marketing authorization for its single-dose chikungunya vaccine, IXCHIQ, extending its approved use to individuals aged 12 years and older across the European Union, Norway, Liechtenstein, and Iceland. As the world’s first licensed chikungunya vaccine, IXCHIQ addresses a significant unmet medical need and is also approved for adults in the United States, Canada, and the United Kingdom, with similar adolescent label extension applications under review in those countries.
- In May 2024, the Ministry of Health and Prevention (MoHAP) urged all pilgrims traveling to Saudi Arabia for Hajj to receive the seasonal influenza vaccine and complete all required immunizations. Pilgrims are advised to ensure they receive the necessary vaccine doses at least 15 days before departure, allowing sufficient time for the vaccines to become effective and provide adequate immunity. Additionally, MoHAP emphasizes that all vaccinations should be documented on the international vaccination card by accredited health centers.

