Oncolytic Viral Therapy Market is Segmented By Virus Type, Application, Mode of Administration, End-User and By Region (North America, Latin America, Europe, Asia Pacific, Middle East, And Africa) – Share, Size, Outlook, And Opportunity Analysis, 2024-2031
Market Overview
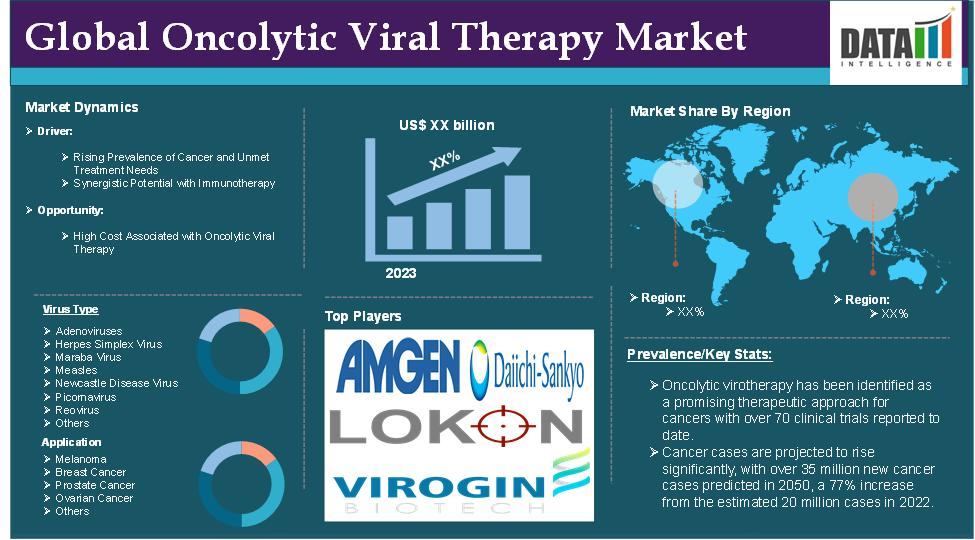
The global oncolytic viral therapy market reached US$ XX billion in 2023 and is expected to reach US$ XX billion by 2031, growing at a CAGR of XX% during the forecast period 2024-2031.
Oncolytic viral therapy is an innovative cancer treatment that uses genetically engineered or naturally occurring viruses to selectively infect, replicate within and ultimately destroy cancer cells while sparing normal, healthy cells. This therapy leverages the unique properties of specific viruses that can target tumor cells, exploiting their distinct environment, altered metabolism and weakened antiviral defenses. Oncolytic viral therapy holds promise for treating both localized and metastatic cancers, offering a new weapon in the fight against cancer, particularly for cases that are resistant to conventional treatments.
The demand for the oncolytic viral therapy market is being driven by several key factors, given the unique mechanisms of oncolytic viral therapy and its potential in cancer treatment. For instance, according to ScienceDirect, oncolytic virotherapy has been identified as a promising therapeutic approach for cancers with over 70 clinical trials reported to date.
Executive Summary
Market Dynamics: Drivers & Restraints
Rising prevalence of cancer and unmet treatment needs
The rising prevalence of cancer and unmet treatment needs are significantly driving the growth of the oncolytic viral therapy market and are expected to drive the market over the forecast period. These factors push demand for innovative therapies like oncolytic viral therapy that can provide effective treatment for challenging cancer cases.
According to the World Health Organization (WHO), cancer cases are projected to rise significantly, with over 35 million new cancer cases predicted in 2050, a 77% increase from the estimated 20 million cases in 2022. This steady increase results in a larger patient population needing diverse treatment options. For cancers such as melanoma, glioblastoma and pancreatic cancer where conventional therapies often fail to provide satisfactory outcomes, there is a high demand for new approaches. Oncolytic viral therapy, with its dual mechanism of selectively infecting cancer cells and stimulating immune responses, addresses the limitations of traditional treatments by offering a targeted approach that spares healthy tissue.
Many cancers, particularly solid tumors and metastatic forms are resistant to chemotherapy and radiation. Furthermore, traditional treatments may have severe side effects, reducing patients’ quality of life and prompting demand for less toxic therapies. Oncolytic viral therapy offers a targeted approach that minimizes damage to healthy cells while activating immune responses against cancer cells, making it a promising option for patients who cannot tolerate existing therapies.
High cost associated with oncolytic viral therapy
Oncolytic viruses require specialized facilities, advanced technology and stringent regulatory compliance, all of which add to production costs. Manufacturing and ensuring the stability of viral therapies are complex and costly processes, making oncolytic viral therapy more expensive than conventional cancer treatments.
For instance, Amgen anticipates the average cost of IMLYGIC therapy to be approximately $65,000, which is costly due to its manufacturing process, involving genetic engineering and purification to ensure safety. These high costs limit its accessibility, especially in regions with limited healthcare funding or infrastructure.
For more details on this report - Request for Sample
Segment Analysis
The global oncolytic viral therapy market is segmented based on virus type, application, mode of administration, end-user and region.
Virus Type:
The herpes simplex virus segment is expected to dominate the global oncolytic viral therapy market share
The herpes simplex virus segment holds a major portion of the oncolytic viral therapy market share and is expected to continue to hold a significant portion of the market share over the forecast period. HSV-based T-VEC (IMLYGIC), developed by Amgen, was the first oncolytic viral therapy approved by the FDA in 2015 for the treatment of melanoma. This approval marked a significant milestone for the oncolytic viral therapy market, establishing herpes simplex virus as a proven and regulatory-accepted virus for clinical use.
The herpes simplex virus has a large genome that can be easily engineered to include therapeutic genes or immune-stimulating factors. This flexibility allows researchers to tailor HSV to different cancers and enhance its tumor-targeting abilities. For instance, in T-VEC, HSV was genetically modified to delete genes that would make it harmful to normal cells, while adding genes to boost immune response within tumors. This selective targeting made it effective in treating cancer cells with reduced adverse effects, a feature that increases its attractiveness in the market.
North America is expected to hold a significant position in the global oncolytic viral therapy market
North America region is expected to hold the largest share in the global oncolytic viral therapy market over the forecast period. North America, especially in the United States has a high prevalence of various cancers, which creates a strong demand for innovative therapies like oncolytic viral therapy.
For instance, according to the CDC, in the United States in 2021, 1,777,566 new cancer cases were reported. In the United States in 2022, 608,366 people died of cancer. In the United States in 2021, 272,454 new breast cancers were reported in females. In the United States in 2021, 141,902 new colorectal cancers were reported. In the U.S. in 2021, 209,500 new lung cancers were reported. This growing cancer burden drives the need for advanced treatment options that can address complex or treatment-resistant cancers.
North America’s regulatory landscape has been favorable for oncolytic viral therapy development, highlighted by the FDA’s approval of T-VEC (talimogene laherparepvec), the first oncolytic viral therapy, in 2015 for melanoma treatment. This approval established a precedent and built confidence in the oncolytic viral therapy market, making North America a leader in oncolytic viral therapy adoption.
Asia Pacific is growing at the fastest pace in the oncolytic viral therapy market
The Asia Pacific region is experiencing the fastest growth in the oncolytic viral therapy market. According to the International Agency for Research on Cancer (IARC), cancer incidence rates in many Asia Pacific countries, particularly China, India and Japan, have been rising due to factors like aging populations, lifestyle changes (e.g., smoking, unhealthy diets), and environmental factors.
For instance, according to the World Economic Forum, Asia Pacific currently accounts for a staggering 45% of all global breast cancer cases and 58% of cervical cancer deaths worldwide and cases of both cancers are expected to rise faster in APAC than in the rest of the world. Unless something changes in Asia, breast cancer cases are anticipated to rise by 21% and cervical cancer incidence is expected to rise by 19% between 2020 and 2030. As a result, the demand for innovative cancer treatments, including oncolytic viral therapy, is growing rapidly to address this increasing burden.
Many Asia Pacific countries have adopted regulatory pathways that allow faster approval and market access for innovative therapies, including oncolytic viral therapy. This has been particularly true in countries like Japan, China and South Korea, where regulatory bodies are working to speed up approval for novel cancer therapies.
For instance, Japan’s PMDA (Pharmaceuticals and Medical Devices Agency) has been proactive in granting fast-track approvals for innovative cancer treatments. In 2015, Japan’s approval of Opdivo (nivolumab), a PD-1 checkpoint inhibitor, set a precedent for accelerated approvals of cancer immunotherapies. This environment has encouraged investment in oncolytic viral therapy, as similar regulatory expedites are applied to oncolytic virus treatments.
Competitive Landscape
The major global players in the oncolytic viral therapy market include Amgen, Inc., Daiichi Sankyo Co., Ltd., Lokon Pharma AB, Virogin Biotech Canada Ltd, KaliVir Immunotherapeutics, Inc., Transgene SA, Vyriad, Inc., Coastar Theapeutics Inc., IconOVir Bio, Inc. Calidi Biotherapeutics, Inc. and among others.
Market Scope
| Metrics | Details | |
| CAGR | XX% | |
| Market Size Available for Years | 2022-2031 | |
| Estimation Forecast Period | 2024-2031 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Virus Type | Adenoviruses, Herpes Simplex Virus, Maraba Virus, Measles, Newcastle Disease Virus, Picornavirus, Reovirus, Vaccinia Virus, Vesicular Stomatitis Virus, Others |
| Application | Melanoma, Breast Cancer, Prostate Cancer, Ovarian Cancer, Lung Cancer, Pancreatic Cancer, Colorectal Cancer, Head and Neck Cancer, Others | |
| Mode of Administration | Intratumoral Injection, Intravenous Infusion, Intracavitary Injection, Others | |
| End-User | Hospitals, Oncology Centers, Ambulatory Surgical Centers, Cancer Research Institutes, Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America and Middle East & Africa | |
Why Purchase the Report?
- To visualize the global oncolytic viral therapy market segmentation based on virus type, application, mode of administration, end-user and region and understand key commercial assets and players.
- Identify commercial opportunities by analyzing trends and co-development.
- Excel data sheet with numerous data points of the oncolytic viral therapy market with all segments.
- PDF report consists of a comprehensive analysis after exhaustive qualitative interviews and an in-depth study.
- Product mapping is available in excel consisting of key products of all the major players.
The global oncolytic viral therapy market report would provide approximately 70 tables, 78 figures and 195 pages.
Target Audience 2023
- Manufacturers/ Buyers
- Industry Investors/Investment Bankers
- Research Professionals
- Emerging Companies


