Industry Outlook
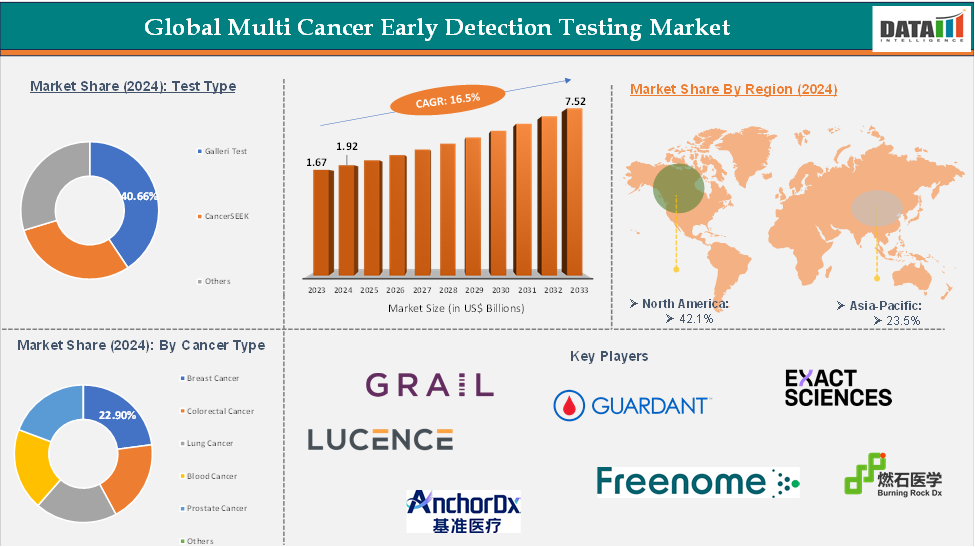
The global multi cancer early detection testing market reached US$ 1.67 billion in 2023, with a rise of US$ 1.92 billion in 2024, and is expected to reach US$ 7.52 billion by 2033, growing at a CAGR of 16.5% during the forecast period 2025-2033.
The global multi-cancer early detection (MCED) testing market is experiencing rapid growth due to the growing global cancer burden and demand for accurate, non-invasive diagnostic solutions. Technological advancements in liquid biopsy, genomics, and AI-driven bioinformatics enable multiple cancer detection from a single blood sample, improving patient outcomes and survival rates. Despite challenges like high testing costs, limited regulatory approvals, and false positives, MCED presents significant opportunities in emerging markets, strategic collaborations, and precision oncology.
Executive Summary
Market Dynamics: Drivers & Restraints
Driver: Rising Global Cancer Prevalence
The global cancer prevalence is driving the multi-cancer early detection (MCED) testing market, as it provides effective and comprehensive screening solutions. Healthcare systems prioritize early detection to improve survival rates and reduce treatment costs. MCED tests, such as liquid biopsy, offer the advantage of identifying multiple cancers at early stages, addressing the growing demand for efficient screening. This trend is driving the adoption and expansion of MCED technologies globally.
For instance, according to American Cancer Society, in 2024, global cancer prevalence remains high, with around 20 million new cases and 9.7 million deaths annually, while 53.5 million people are living within five years of a cancer diagnosis. Lung, breast, and colorectal cancers are the most common, and the lifetime risk of cancer is approximately 1 in 5 people. Rising cases, driven by aging populations and lifestyle factors, are fueling demand for early detection solutions like multi-cancer early detection (MCED) testing.
Restraint: Improving Sensitivity and Specificity
Current MCED tests need improvement, particularly for stage I cancer detection. Novel biomarkers and technologies like cfDNA methylation patterns and miRNAs, ultrasensitive sequencing, and PCR can increase sensitivity. Understanding the causes of false positives in healthy individuals and addressing physiological changes like aging, lifestyle habits, and inflammation is crucial for cancer-related biomarker effects.
For more details on this report, Request for Sample
Segment Analysis
The global multi cancer early detection testing market is segmented based on test type, cancer type, end user, and region.
Test Type:
The galleri test segment the expected to have 40.66% of the multi cancer early detection testing market share.
The Galleri test, developed by GRAIL, is a highly advanced blood-based screening solution for cancer detection. It uses next-generation sequencing and methylation pattern analysis to detect over 50 types of cancer from a single blood draw, often early before symptoms appear. Galleri's strong clinical validation, partnerships with healthcare providers, and integration into large-scale screening programs drive market adoption and accelerate the shift towards proactive and precision oncology.
For instance, GRAIL, a healthcare company, and Quest Diagnostics have announced a program to enhance provider access to GRAIL's Galleri multi-cancer early detection (MCED) test. The initial phase of the program allows providers to order the test directly from GRAIL through the Quest Diagnostics connectivity system. This system allows providers in the US to order and receive laboratory test reports electronically through Quest's Quanum laboratory portal and over 900 electronic health record (EHR) systems.
Geographical Analysis
The North America global multi cancer early detection testing market was valued at 42.1% market share in 2024
North America's Multi-Cancer Early Detection (MCED) Testing Market is thriving due to high cancer burden, robust healthcare infrastructure, and advanced diagnostics adoption. The region benefits from R&D investments, reimbursement frameworks, and awareness of early cancer detection benefits. Leading developers like GRAIL, Exact Sciences, and Guardant Health, along with regulatory support for innovative screening technologies, accelerate market growth. Rising demand for personalized medicine and liquid biopsy-based tests further strengthens North America's growth hub.
For instance, in June 2025, Guardant Health, a precision oncology company, received Breakthrough Device designation from the U.S. Food and Drug Administration for its Shield multi-cancer detection test. The methylation-based blood test is used for screening multiple cancer types in individuals aged 45 or older, who are at average risk for cancer.
Major Key Players
The major players in the multi cancer early detection testing market include GRAIL, Guardant Health, Exact Sciences , Burning Rock Biotech, Lucence Health, Foundation Medicine (Roche), AnchorDx, Freenome, and Niramai among others.
Key Developments
In July 2025, Fred Hutch Cancer Center launched Vanguard Study, a national study evaluating multi-cancer detection (MCD) blood tests for early detection of cancer in individuals aged 45 to 75, aiming to improve treatment outcomes.
In August 2024, Exact Sciences announced that the first patient has joined its Multi-Cancer Early Detection Falcon Registry Real-World Evidence study at Baylor Scott & White, the largest not-for-profit health system in Texas.
Report Scope
Metrics | Details | |
CAGR | 16.5% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Test Type | Galleri Test, CancerSEEK, Others |
Cancer Type | Breast Cancer, Colorectal Cancer, Lung Cancer, Blood Cancer, Prostate Cancer and others. | |
| End User | Hospitals & Clinics, Diagnostic Laboratories, Academic & Research Institutes |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global multi cancer early detection testing market report delivers a detailed analysis with 70 key tables, more than 61 visually impactful figures, and 195 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more pharmaceuticals-related reports, please click here