Mesenchymal Stem Cells Market Size
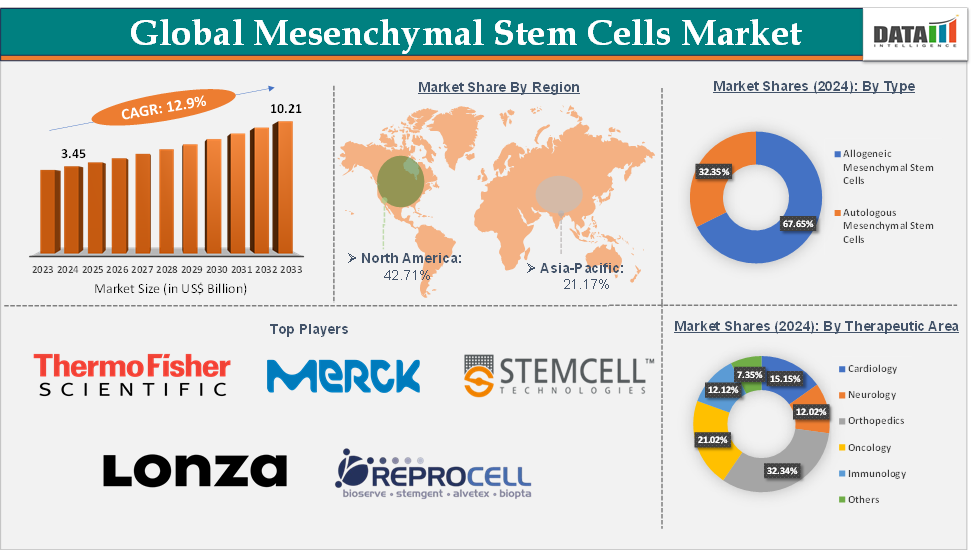
Mesenchymal Stem Cells Market Size reached US$ 3.45 Billion in 2024 and is expected to reach US$ 10.21 Billion by 2033, growing at a CAGR of 12.9% during the forecast period 2025-2033.
Mesenchymal Stem Cells Market Overview
The global mesenchymal stem cells market has witnessed robust expansion in recent years, driven primarily by increasing adoption of cell-based therapies, regenerative medicine applications, and rising incidences of chronic diseases. North America will likely maintain its leadership position, but Asia-Pacific’s rapid growth trajectory underscores shifting epicenters of production and clinical adoption. With major players expanding their product portfolios, pursuing strategic alliances, and navigating evolving reimbursement landscapes, the market is well-positioned to revolutionize treatment paradigms across a range of degenerative and chronic diseases over the next decade.
Executive Summary
For more details on this report – Request for Sample
Mesenchymal Stem Cells Market Dynamics: Drivers & Restraints
The rising adoption of mesenchymal stem cells for various diseases is significantly driving the market growth
Each of these real-world applications has pushed biotech firms and CMOs to scale up production capacity, invest in new donor‐screening pipelines, and secure broader regulatory clearances. As more therapeutic areas validate mesenchymal stem cells' efficacy, end users are allocating larger budgets to MSC acquisition, driving the overall mesenchymal stem cells market growth into double-digit percentages year over year. Overall, major market players and emerging players are developing various MSC products for various disease treatments, which is driving the market growth.
For instance, in February 2025, Mesoblast Limited announced that its recently approved product Ryoncil (remestemcel-L) is being highlighted at the 2025 Transplantation & Cellular Therapy Tandem Meetings of the American Society for Transplantation and Cellular Therapy (ASTCT) and the Center for Blood and Marrow Transplant Research (CIBMTR). Ryoncil was approved in December 2024 by the United States Food and Drug Administration (FDA) for steroid-refractory acute graft-versus-host disease (SR-aGvHD) in pediatric patients 2 months and older, becoming the first mesenchymal stromal cell (MSC) therapy approved in the U.S. for any indication.
Similarly, in November 2024, Global Institute of Stem Cell Therapy and Research, Inc., known as GIOSTAR, announced that the United States Food and Drug Administration (FDA) cleared its investigational new drug (IND) application to start a Phase 2 clinical trial for DT2-SCT. The Company’s novel approach using autologous mesenchymal stem cells to alleviate the disease-caused damage in diabetics offers a new hope to address the sufferings in diabetes patients without many side effects.
High costs associated with mesenchymal stem cell therapy are hampering the growth of the mesenchymal stem cells market
The high cost associated with mesenchymal stem cell therapy is a significant barrier that hampers the growth of the mesenchymal stem cells market. Despite promising applications, mesenchymal stem cell therapies remain largely inaccessible to a broad range of patients due to their expense, limiting adoption rates, and slowing market expansion.
For instance, according to the Stemaid Institute, mesenchymal stem cell therapy treatment can range from $20,000 to $100,000 for whole-body treatment. Additionally, DVC Stem's IRB-approved mesenchymal stem cell protocol is currently priced at $25,000. According to the Frontiers case analysis for allogeneic mesenchymal stem cells, one administration of allogeneic mesenchymal stem cells had an expected total cost of $13,536.
Mesenchymal Stem Cells Market, Segment Analysis
The global mesenchymal stem cells market is segmented based on type, source, therapeutic area, application, end-user, and region.
The orthopedics segment from the therapeutic area is expected to hold 32.34% of the market share in 2024 in the mesenchymal stem cells market
Mesenchymal stem cells are especially effective in treating conditions like various arthritis, bone fractures, and cartilage damage due to their unique ability to differentiate into bone (osteocytes) cells, which makes them valuable in regenerative medicine for musculoskeletal disorders. The FDA and EMA have granted designations for multiple mesenchymal stem cell candidates targeting osteoarthritic cartilage repair. These regulatory approvals of various mesenchymal stem cells for orthopedic clinical trials and treatments boost the segment's growth.
For instance, in March 2025, FDA authorized Hope Biosciences’ adipose-derived mesenchymal stem cells (HB-adMSCs) for use in the first pediatric clinical trial, to evaluate if intravenous infusion affects signs and symptoms of oligoarticular or polyarticular juvenile idiopathic arthritis (JIA) and improve quality of life in suffering children aged 2 – 16 years old. Hope Biosciences’ proprietary cell therapy product has been previously employed in multiple conditions similar or related to JIA, including rheumatoid arthritis, chronic musculoskeletal pain, severe osteoarthritis, psoriatic arthritis, and lupus. These protocols concluded that HB-adMSCs are safe and can create significant improvements in pain levels and functionality.
Mesenchymal Stem Cells Market Geographical Analysis
North America is expected to dominate the global mesenchymal stem cells market with a 42.71% share in 2024
North America, particularly the United States and Canada, has some of the most advanced healthcare infrastructures in the world, including cutting-edge hospitals, specialized clinics, and research institutions. This infrastructure supports the adoption of innovative therapies like mesenchymal stem cells in treating various conditions, including orthopedic, autoimmune, and degenerative diseases.
According to the Centers for Disease Control and Prevention (CDC), an estimated 129 million people in the US have at least 1 major chronic disease. The National Health Council indicates that autoimmune diseases affect approximately 50 million Americans. The National Institutes of Health (NIH) estimates that they collectively affect between 5% and 8% of the U.S. population. This high prevalence of chronic diseases has created a substantial demand for mesenchymal stem cells in North America, especially in the United States.
Asia-Pacific is growing at the fastest pace in the mesenchymal stem cells market, holding 21.17% of the market share
Asia Pacific countries, including China, India, Japan, and South Korea, have significantly improved their healthcare infrastructures in recent years. With the rising demand for advanced medical technologies, these countries are becoming key players in the mesenchymal stem cell therapy market.
The region is seeing a surge in both public and private sector investment in biotechnology and regenerative medicine. For instance, according to the University of York, in China, annual investment in stem cell research presently stands somewhere between US$4 and 10 million, with 300 researchers working in 30 separate institutions, these figures are projected to increase dramatically. Similarly, in India, the government has been supporting biotech initiatives, leading to a rise in clinical trials and stem cell research.
Asia Pacific has a large and growing population of people suffering from chronic diseases such as osteoarthritis, diabetes, cardiovascular diseases, and neurodegenerative disorders. According to the ESCAP, in Asia Pacific, the number of older persons is projected to more than double, from 630 million in 2020 to about 1.3 billion by 2050. With an aging population in Asia Pacific, the demand for regenerative therapies, including mesenchymal stem cells, is escalating.
Mesenchymal Stem Cells Market Top Companies
Top companies in the mesenchymal stem cells market include Thermo Fisher Scientific Inc., Merck KGaA, Lonza Ltd, Bio-Techne, STEMCELL Technologies Inc., REPROCELL Inc., AcceGen, PromoCell GmbH, Celprogen Inc., and Cyagen Biosciences Inc., among others.
Emerging Players
The emerging players in the mesenchymal stem cells market include Mesoblast Ltd., Celltex Therapeutics, Beike Biotechnology, Athersys, Inc., and Cynata Therapeutics, among others.
Mesenchymal Stem Cells Market Key Developments
In January 2025, the Food and Drug Administration (FDA) approved the first mesenchymal stromal cell (MSC) therapy, RYONCIL, indicated for steroid-refractory acute graft-versus-host disease (SR-aGVHD) in children two months and older. This milestone in regenerative medicine was developed and patented by Osiris Therapeutics.
In December 2024, Eterna Therapeutics announced it will investigate the ability of its lead iMSC-based cell therapy (ERNA-101) to induce and modulate antitumor immunity in ovarian cancer and breast cancer models through a sponsored research agreement with The University of Texas MD Anderson Cancer Center. ERNA-101 is Eterna’s proprietary allogenic IL-7 and IL-15-secreting induced pluripotent stem cell (iPSC) derived mesenchymal stem cells (iMSC) product candidate.
In September 2024, Angela Caglia, Master Esthetician and skin care formulator, debuted her self-named skin care brand's Cellular Collection, which leverages human mesenchymal stem cells (MSCs) byproducts in skin care. The first product in this line, Cell Forté Serum, utilizes the world's first patented human-derived stem cell technology byproduct, marking a significant innovation in non-invasive anti-aging solutions.
Market Scope
Metrics | Details | |
CAGR | 12.9% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Type | Allogeneic Mesenchymal Stem Cells, Autologous Mesenchymal Stem Cells |
Source | Bone Marrow, Adipose Tissue, Umbilical Cord, Dental Pulp, Placenta, Periosteum, Others | |
Therapeutic Area | Cardiology, Neurology, Orthopedics, Oncology, Immunology, and Others | |
Application | Regenerative Medicine, Disease Modelling, Drug Development & Discovery, Stem Cell Banking, Tissue Engineering, Toxicology Studies, Others | |
End-User | Hospitals, Specialty Clinics, Academic and Research Institutes, Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global mesenchymal Stem Cells market report delivers a detailed analysis with 75+ key tables, more than 80+ visually impactful figures, and 187 pages of expert insights, providing a complete view of the market landscape.


