Overview
Lung cancer is a type of cancer that begins in the lungs, which are the two spongy organs in the chest responsible for breathing. It is characterized by the uncontrolled growth of abnormal cells in the lung tissue, which can form a tumor. Over time, these cancerous cells can spread (metastasize) to other parts of the body. Treatment options depend on the type, stage, and location of the lung cancer, as well as the patient's overall health.
Executive Summary
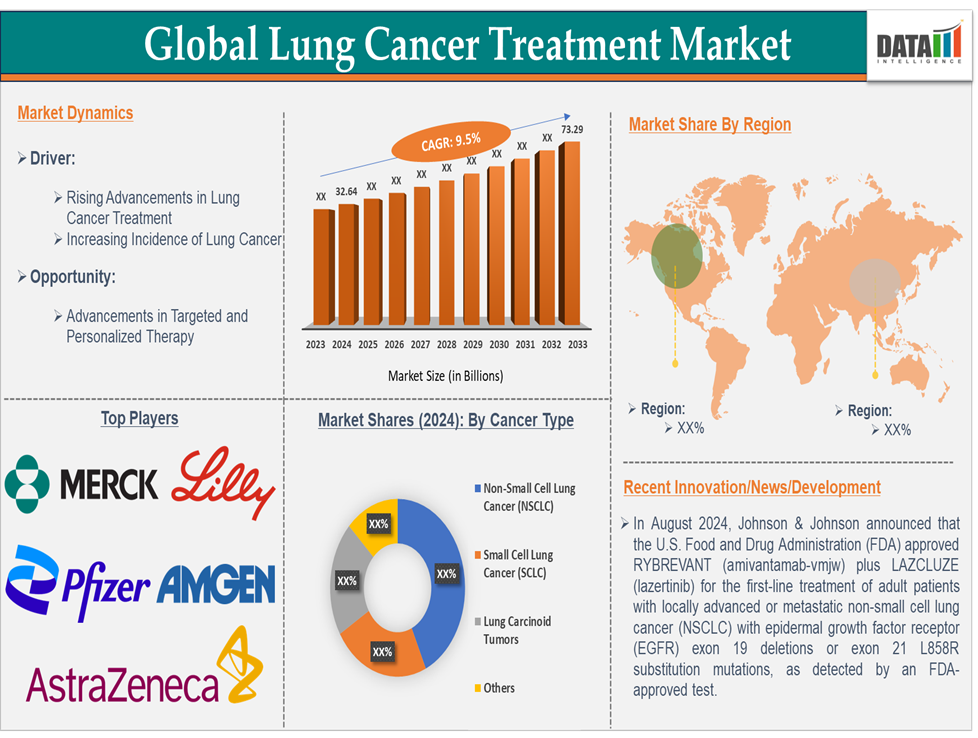
Market Dynamics: Drivers & Restraints
Rising advancements in lung cancer treatment are significantly driving the market growth
Targeted therapies focus on specific genetic mutations and molecular changes in cancer cells, making treatments more precise and effective. These therapies are less toxic than traditional chemotherapy and are used to treat cancers with specific mutations. For instance, Osimertinib (Tagrisso) is a targeted therapy used to treat non-small cell lung cancer (NSCLC) with an EGFR mutation. It is more effective than traditional chemotherapy, leading to better survival outcomes and fewer side effects. In 2024, Tagrisso generated 6,580 million in sales, demonstrating the market's growing demand for targeted therapies.
Immunotherapy boosts the body’s immune system to fight cancer cells. It has shown promising results, particularly in advanced stages of lung cancer, where it can help in prolonging life and even achieving remission. For instance, Pembrolizumab (Keytruda) and Nivolumab (Opdivo) are immune checkpoint inhibitors that block the PD-1 protein, allowing the immune system to target cancer cells more effectively. These drugs have been game-changers for patients with advanced NSCLC, leading to a significant market shift towards immunotherapy.
These advancements have led to a shift from traditional, broad-based treatments to more targeted, personalized therapies. This change not only improves treatment outcomes but also creates a larger market for new therapies and personalized treatment options. As more patients benefit from these innovations, the demand for advanced lung cancer treatments continues to rise, driving significant market growth.
Side effects associated with lung cancer treatments are hampering the market growth
Side effects associated with lung cancer treatments can hamper market growth by discouraging patients from undergoing treatment, limiting the effectiveness of therapies, and increasing the healthcare burden. While advancements in lung cancer treatments have improved survival rates, many therapies still come with significant side effects that can affect the quality of life, increase treatment costs, and slow patient adoption of newer therapies.
Chemotherapy is a standard treatment for lung cancer, especially in advanced stages. However, it is associated with several severe side effects, including nausea, fatigue, hair loss, immune suppression, and anemia. These side effects can significantly reduce patients' quality of life and lead to treatment discontinuation or delays, hampering the overall effectiveness of chemotherapy in managing the disease.
For instance, Cisplatin and Carboplatin, two common chemotherapy drugs used to treat lung cancer, can cause severe nausea, vomiting, and kidney damage. These side effects make chemotherapy challenging for patients, leading to hesitancy in using these drugs, especially for older or frail patients.
While targeted therapies are more specific and less toxic than chemotherapy, they still come with side effects that can discourage patients from continuing treatment. These side effects can include skin rashes, diarrhea, liver problems, and fatigue. Even though targeted therapies are highly effective in treating cancers with specific mutations, the adverse reactions can affect the market's growth potential.
Epidemiology Analysis
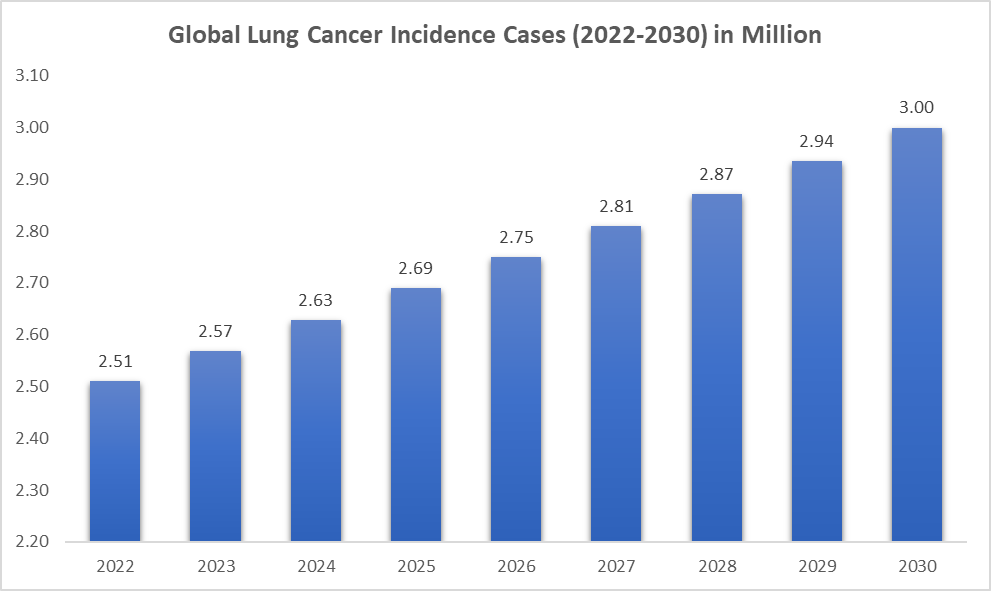
The incidence of lung cancer is rising. As per DataM intelligence estimates, nearly 2.63 million prevalent cases are estimated worldwide in 2024. The region with the highest prevalence is the Asia-Pacific, accounting for 1.66 million cases. Lung cancer is one of the most common and lethal cancers worldwide. Its epidemiology is influenced by various factors, including smoking habits, environmental exposures, genetic predispositions, and access to early detection and treatment. While advancements in treatment have improved survival rates, challenges such as late-stage diagnosis, high treatment costs, and regional disparities in care continue to impact outcomes. Early detection, reduced smoking rates, and improvements in personalized treatments hold the key to reducing the burden of lung cancer worldwide.
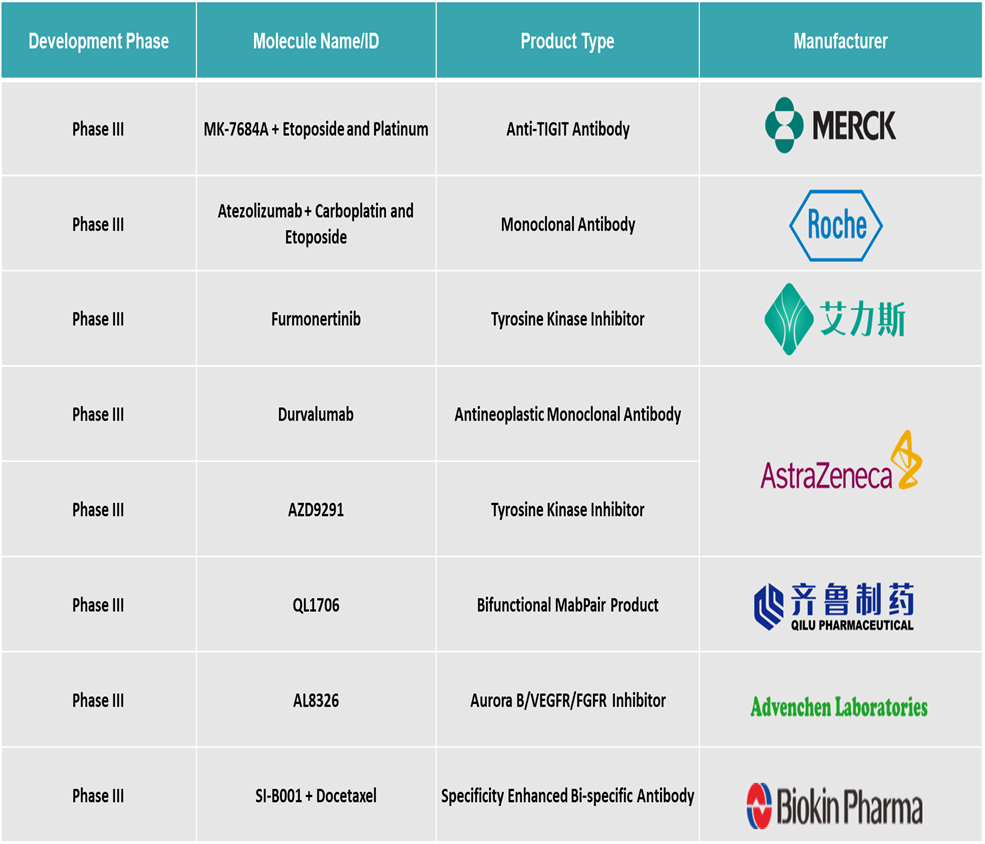
Pipeline Analysis
Top phase III pipeline products for lung cancer:
Segment Analysis
The global lung cancer treatment market is segmented based on cancer type, treatment type, and region.
Cancer Type:
The Non-Small Cell Lung Cancer (NSCLC) segment is expected to dominate the lung cancer treatment market with the highest market share
NSCLC offers a variety of treatment options, including surgery, chemotherapy, targeted therapies, immunotherapy, and a combination of these. The diversity of treatment methods caters to different stages (early vs. advanced) and specific genetic mutations or biomarkers within NSCLC, thus increasing its market potential. Many market players are continuously performing clinical trials for better treatment of NSCLC, which further boosts the segment growth.
For instance, in August 2024, Bayer announced that the first patient has been enrolled in the global Phase III SOHO-02 trial, an open-label, randomized, multicenter clinical trial, assessing the efficacy and safety of investigational agent BAY 2927088 as first-line therapy in patients with advanced non-small cell lung cancer (NSCLC), whose tumors have activating HER2 mutations.
A major portion of lung cancer research and pharmaceutical R&D investments is focused on NSCLC, given its high prevalence and complex biology. This results in the continual development of newer drugs and treatment approaches, including combination therapies that increase treatment efficacy. Thus, rising FDA approvals for NSCLC are further driving the segment demand.
For instance, in August 2024, Johnson & Johnson announced that the U.S. Food and Drug Administration (FDA) approved RYBREVANT (amivantamab-vmjw) plus LAZCLUZE (lazertinib) for the first-line treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test.
Geographical Analysis
North America is expected to hold a significant position in the global Lung cancer treatment market with the highest market share
North America, particularly the United States, has a significant burden of lung cancer cases, which drives the demand for lung cancer treatments. Lung cancer remains one of the most common cancers in the region, largely due to high smoking rates (though smoking rates have been declining), air pollution, and other risk factors like radon exposure.
For instance, according to the American Cancer Society’s estimates for lung cancer in the US for 2025 are about 226,650 new cases of lung cancer (110,680 in men and 115,970 in women) and about 124,730 deaths from lung cancer (64,190 in men and 60,540 in women). This high incidence leads to increased demand for treatments and contributes to the region's large share of the global lung cancer treatment market.
The U.S. Food and Drug Administration (FDA) is one of the fastest regulators in terms of approving new therapies, including cancer treatments. FDA approval is often seen as a critical step in the global market entry for new treatments. North America benefits from early access to cutting-edge therapies as they are approved, enabling patients to access the most effective treatment options.
For instance, in December 2024, Xcovery Holdings, Inc., an oncology-focused pharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) approved ensartinib (Ensacove, Xcovery Holdings, Inc.) for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive locally advanced or metastatic non-small cell lung cancer (NSCLC). This approval marks an important advancement in providing a new first-line option for patients with ALK-positive NSCLC.
Competitive Landscape
Top companies in the lung cancer treatment market include Merck & Co., Inc., Pfizer Inc., AstraZeneca, Amgen Inc., Eli Lilly and Company, Bristol-Myers Squibb Company, Takeda Pharmaceuticals U.S.A., Inc., Amneal Pharmaceuticals LLC., Genentech USA, Inc., Rigel Pharmaceuticals, Inc., Daiichi Sankyo, Inc. and among others.
Scope
| Metrics | Details | |
| CAGR | 9.5% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Cancer Type | Non-Small Cell Lung Cancer (NSCLC), Small Cell Lung Cancer (SCLC), Lung Carcinoid Tumors, and Others |
| Treatment Type | Chemotherapy, Radiation Therapy, Targeted Therapy, Surgery, and Others | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials, product pipelines, and forecasts upcoming advancements in medical devices and pharmaceuticals.
- Product Performance & Market Positioning: Analyze product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: Covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyzes competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient product delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global lung cancer treatment market report delivers a detailed analysis with 57 key tables, more than 46 visually impactful figures, and 168 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2024
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.


