Global Lung Cancer Therapeutics Market: Industry Outlook
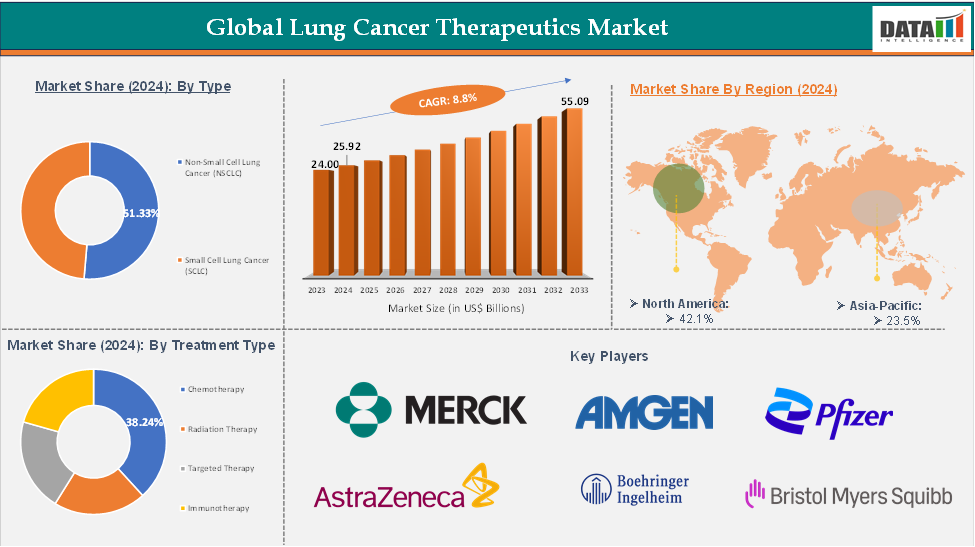
The global lung cancer therapeutics market reached US$ 24.00 Billion in 2023, with a rise of US$ 25.92 Billion in 2024 and is expected to reach US$ 55.09 Billion by 2033, growing at a CAGR of 8.8% during the forecast period 2025-2033.
The global lung cancer therapeutics market is experiencing significant growth due to the increasing burden of lung cancer, the leading cause of cancer-related deaths worldwide. Factors such as smoking habits, air pollution, occupational hazards, and aging populations are contributing to this growth. The demand for innovative treatment options, including targeted therapies and immunotherapies, is increasing.
Pharmaceutical companies and research institutes are investing in R&D to discover new molecules, biomarkers, and gene-specific therapies to improve survival rates and quality of life. Governments and healthcare organizations are expanding lung cancer screening programs and enhancing access to treatment, particularly in high-incidence regions. The market is poised to benefit from personalized treatment solutions and next-generation therapeutics, transforming patient outcomes globally.
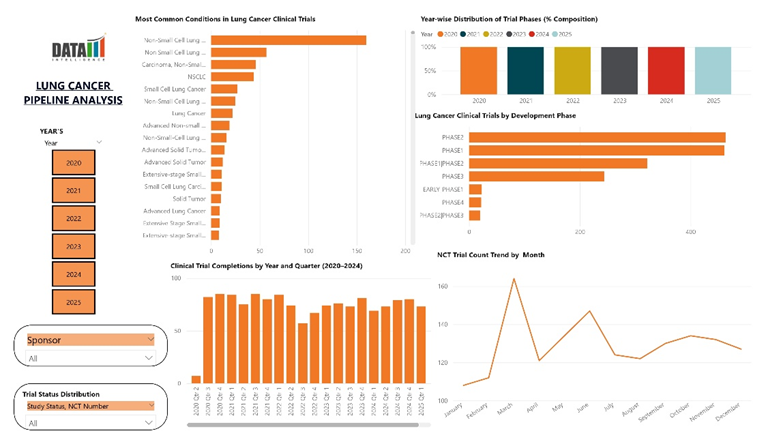
Pipeline Analysis in Dashboard View:
Global Lung Cancer Therapeutics Market: Executive Summary
Global Lung Cancer Therapeutics Market Dynamics: Drivers & Restraints
Driver: Rising incidence of lung cancer
Lung cancer is the leading cause of cancer mortality globally. This trend is particularly alarming in emerging economies due to urbanization, industrialization, and tobacco use. As populations age, the risk of developing lung cancer increases, putting pressure on healthcare systems to develop effective and scalable treatment solutions.
The increasing number of cases has led to a demand for improved diagnostic tools and therapeutic interventions, prompting pharmaceutical companies to expand research into advanced treatment modalities. Public health awareness and government-backed initiatives to fund screening and early diagnosis programs contribute to market expansion. The need for timely and effective treatments is driving robust growth in the global therapeutics market. For instance, in 2024, lung cancer remains a significant global health concern, accounting for 12.4% of all new cancer cases and 1.8 million deaths annually, with approximately 2.5 million new cases reported.
Driver: Advancements in immunotherapy and targeted therapies
The use of immunotherapy and targeted therapies has significantly transformed the treatment of lung cancer, enhancing survival rates and quality of life. Traditional chemotherapy, which has limitations in efficacy and side effects, is still widely used. Immunotherapies like immune checkpoint inhibitors have shown success in recognizing and attacking cancer cells, leading to longer survival.
Targeted therapies, targeting specific genetic mutations, offer a personalized approach, minimizing harm to healthy cells and improving treatment outcomes. These therapies are particularly effective in non-small cell lung cancer (NSCLC), which accounts for most cases. The availability of genetic testing and biomarker identification has made personalized medicine a growing trend. The market is experiencing a surge in approvals and launches of novel agents, reshaping the commercial dynamics of the lung cancer therapeutics market.
Restraint: High cost of advanced therapies
The global lung cancer therapeutics market faces significant challenges due to the high cost of modern treatment options, particularly targeted therapy and immunotherapy. These therapies, while clinically effective, are financially burdensome for patients and healthcare systems, particularly in countries with limited reimbursement coverage. In the U.S., the cost of a full course of immunotherapy can be hundreds of thousands of dollars per year, making it inaccessible to many uninsured or underinsured patients.
The cost of diagnostic tests for targeted therapy, such as genomic profiling and biomarker testing, exacerbates the financial burden. Despite the potential benefits of generic drugs and biosimilars, the current market dynamics favor expensive branded drugs with limited competition, limiting patient access and hindering market penetration, especially in emerging markets.
Opportunity: Growth in early detection and liquid biopsy technologies
The lung cancer therapeutics market is experiencing a significant opportunity in the development and adoption of early detection technologies, particularly liquid biopsies and biomarker-based diagnostics. Early-stage lung cancer is often asymptomatic, leading to limited treatment options and high mortality rates. Advancements in molecular diagnostics and non-invasive testing methods, such as liquid biopsies, are paving the way for earlier and more accurate detection.
These technologies help identify lung cancer at a treatable stage, guide targeted therapies, and monitor treatment response. Companies investing in AI-driven screening tools and personalized biomarker panels are capitalizing on this unmet need. Governments and healthcare organizations are funding pilot programs and research initiatives to deploy these technologies at scale. As regulatory approvals increase and costs decrease, early detection tools are expected to become integral to lung cancer management.
For more details on this report, Request for Sample
Global Lung Cancer Therapeutics Market Segment Analysis
The global lung cancer therapeutics market is segmented based on disease type, treatment type, route of administration, distribution channel, and region.
Treatment Type:
The chemotherapy segment from the treatment type is expected to have 38.24% of the lung cancer therapeutics market share.
Chemotherapy remains a crucial part of lung cancer treatment, especially for advanced stages or those who cannot qualify for newer therapies. It is especially important for small cell lung cancer (SCLC), which is more aggressive and less responsive to targeted options. In many developing countries, chemotherapy remains the most accessible and cost-effective treatment option due to limited infrastructure and affordability constraints.
In developed nations, chemotherapy is often used in combination with immunotherapy and radiation to enhance therapeutic efficacy. The broad applicability of chemotherapy across different lung cancer stages and subtypes ensures continued demand for these agents. Research into optimizing dosing schedules, minimizing side effects, and improving combination therapies is helping to sustain its relevance.
For instance, Glenmark Pharmaceuticals launched Tevimbra, a lung cancer treatment drug developed by global oncology leader BeiGene, in India, marking its first foray into immune-oncology in the country. The approval from the Central Drugs Standard Control Organisation marks a significant milestone in the company's innovative oncology portfolio.
Global Lung Cancer Therapeutics Market - Geographical Analysis
The North America global lung cancer therapeutics market was valued at 10.91 Billion in 2024
North America leads the global lung cancer therapeutics market due to its advanced healthcare infrastructure, high awareness levels, and early adoption of innovative treatments. The US has a well-established regulatory environment, with the FDA fast-tracking the approval of breakthrough therapies and expanding indications for existing drugs.
Major pharmaceutical companies and research institutions foster an environment of continuous innovation, leading to a diverse pipeline of novel lung cancer therapies. Reimbursement policies, particularly under Medicare and private insurers, ensure patient access to high-cost treatments.
For instance, in May 2025, the FDA granted fast-track designation to ZL-1310, a promising new treatment for ES-SCLC patients, allowing the FDA to expedite the review process with Zai Lab, the drug's developer.
The Asia-Pacific global lung cancer therapeutics market was valued at 6.09 Billion in 2024
The Asia-Pacific region is experiencing a rapid growth in lung cancer therapeutics due to factors such as increased tobacco consumption, urban air pollution, and improved healthcare infrastructure. Countries like China, India, Japan, and South Korea are experiencing a surge in lung cancer cases, prompting governments to invest in awareness campaigns and screening programs. As healthcare access improves in rural and semi-urban regions, more patients are being diagnosed and treated, driving demand for both traditional and advanced therapeutics.
The rise of domestic pharmaceutical manufacturers and affordable generic drugs has made treatments more accessible to a broader population. Global pharmaceutical companies are also entering strategic partnerships with local players to tap into this underserved market. The region is also becoming a preferred destination for clinical trials due to its large patient pool, lower operational costs, and regulatory reforms.
For instance, in May 2025, Johnson & Johnson and Yuhan Corp. launched a chemo-free dual therapy for Japanese lung cancer patients with common EGFR mutations. The combination of Rybrevant and Yuhan's Lazcluze is intended for first-line treatment of unresectable, advanced, or recurrent NSCLC with EGFR exon 19 deletions or exon 21 L858R mutations.
Global Lung Cancer Therapeutics Market – Major Players
The major players in the lung cancer therapeutics market include Pfizer, Merck & Co., Amgen Inc., AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb Company, Novartis AG, Eli Lilly and Company, Hoffmann-La Roche, and AbbVie, Inc., among others.
Global Lung Cancer Therapeutics Market – Key Developments
In June 2025, Glenmark Pharmaceuticals launched Tevimbra, a lung cancer treatment drug developed by global oncology leader BeiGene, in India, marking its first foray into immune-oncology in the country. The approval from the Central Drugs Standard Control Organisation marks a significant milestone in the company's innovative oncology portfolio.
In May 2025, Dana-Farber Cancer Institute researchers launched a first-of-its-kind study using a homegrown blood test to identify individuals at high risk of lung cancer who have never used tobacco. The team, led by principal investigator Narjust Florez, MD, is designed to address rising rates of lung cancer in younger people and prevalence in individuals of Asian or Hispanic/Latinx descent who have never used tobacco. The study aims to offer complimentary lung cancer screening to those who have never used tobacco.
Global Lung Cancer Therapeutics Market: Scope
Metrics | Details | |
CAGR | 8.8% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Type | Non-Small Cell Lung Cancer (NSCLC), Small Cell Lung Cancer (SCLC) |
Treatment Type | Chemotherapy, Radiation Therapy, Targeted Therapy, Immunotherapy | |
| Route of Administration | Oral, Intravenous |
| Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
DMI Insights:
The global lung cancer therapeutics market is primarily driven by the rising global prevalence of lung cancer, advancements in immunotherapy and targeted therapies, and increasing awareness around early diagnosis. As healthcare systems continue to embrace precision medicine and adopt cutting-edge diagnostic tools, the market is expected to witness strong growth in the coming years.
The expanding elderly population, growing investments in oncology R&D, and favorable regulatory support further strengthen market potential. With a robust pipeline of next-generation therapies and increasing access in emerging economies, the market is poised for sustained expansion through 2033.
The global lung cancer therapeutics market report delivers a detailed analysis with 70 key tables, more than 61 visually impactful figures, and 205 pages of expert insights, providing a complete view of the market landscape.
Suggestions for Related Report
For more pharmaceutical-related reports, please click here

