Executive Summary
The exosome therapeutics market is rapidly emerging as one of the most transformative areas in biopharmaceutical innovation, with applications spanning oncology, neurology, cardiovascular disease, regenerative medicine, and inflammatory disorders. Exosomes, as naturally derived extracellular vesicles, offer unique advantages in targeted drug delivery, cell-to-cell communication, and disease modulation, positioning them as next-generation therapeutic modalities. A growing clinical pipeline, including multiple Phase I/II and early Phase III trials, is exploring its potential in addressing unmet medical needs where conventional therapies have shown limited efficacy. Advances in scalable biomanufacturing, engineering of exosomes for improved targeting, and standardization of safety and quality protocols are accelerating their path toward commercialization. With the rising investment from both pharmaceutical companies and venture capital and expanding academic-industry collaborations, the market is expected to evolve into a structured, evidence-driven ecosystem over the next decade.
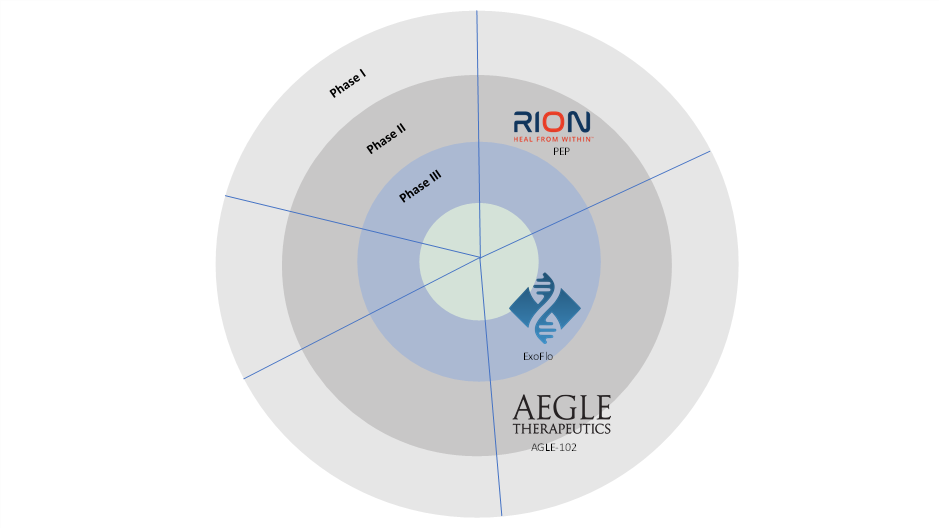
Pipeline Analysis
List of Key Companies
The major and key players in the exosome therapeutics pipeline include
- Rion Inc.
- AEGLE Therapeutics
- Direct Biologics LLC
- Kimera Labs
Key Developments
| Molecule | Company | Clinical Phase | Indication |
| PEP | Rion Inc. | Phase II | Diabetic Foot Ulcers (DFU) |
| AGLE-102 | AEGLE Therapeutics | Phase I/II | Epidermolysis Bullosa (EB) |
| ExoFlo | Direct Biologics LLC | Phase III | Acute Respiratory Distress Syndrome (ARDS) |
- In May 2025, Kimera Labs, a leader in therapeutic exosome biotechnology, announced its selection as a semifinalist in the prestigious $101 million XPRIZE Healthspan competition. This achievement highlights Kimera’s pioneering efforts in advancing exosome-based therapeutics designed to meaningfully extend human healthspan and promote longer, more active lives.
- In July 2023, Direct Biologics, LLC, a late-stage biotechnology company advancing a regenerative medicine platform based on extracellular vesicles (EVs) derived from bone marrow mesenchymal stem cells (BM-MSCs), announced positive outcomes from its Phase 2 clinical trial (NCT04493242) of ExoFlo. The trial in hospitalized adult COVID-19 patients with moderate-to-severe acute respiratory distress syndrome (ARDS) demonstrated strong safety results along with significant efficacy benefits.
Future Perspectives and Conclusion
The exosome therapeutics pipeline is set for transformative advancement as research momentum and clinical development accelerate. With expanding applications across oncology, neurology, regenerative medicine, cardiovascular, and inflammatory disorders, the next wave of innovation is expected to emphasize personalized, disease-specific therapies powered by engineered exosomes and advanced delivery systems. Ongoing Phase I/II studies and progressing Phase III trials highlight the promise of exosome-based candidates to achieve durable efficacy, improved safety, and novel mechanisms of action in areas of high unmet medical need. Continued progress in scalable manufacturing, dosing optimization, and regulatory standardization is shaping more reliable and commercially viable therapeutic models. In parallel, growing industry collaborations, investment inflows, and supportive regulatory pathways are reinforcing clinical translation and market readiness. Over the coming years, the exosome therapeutics pipeline is likely to evolve into a structured, evidence-driven ecosystem, delivering targeted, accessible, and next-generation treatment solutions that position exosomes as a mainstream modality in modern medicine.