CRISPR-Based Diagnostics Market Size
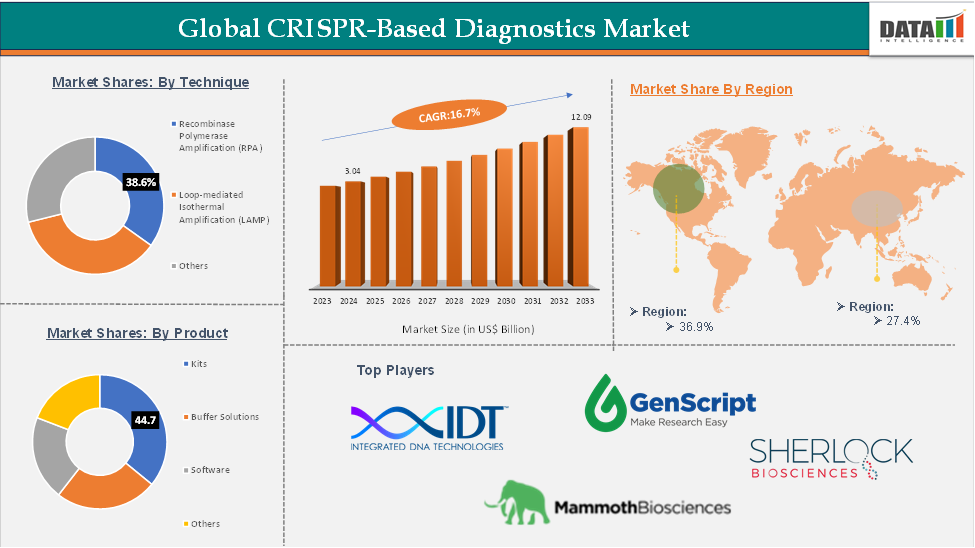
CRISPR-Based Diagnostics Market size reached US$ 3.04 Billion in 2024 and is expected to reach US$ 12.09 Billion by 2033, growing at a CAGR of 16.7% during the forecast period 2025-2033.
CRISPR-based diagnostics have emerged as promising tools capable of revolutionizing the field of molecular diagnostics. CRISPR enzymes can be useful in biomedical applications to recognize biomarkers of infectious and non-infectious diseases, as well as cancer and many other diseases that involve a nucleic acid sequence.
Market growth is being driven by the rising global burden of infectious diseases such as tuberculosis, dengue, and emerging viral threats, alongside a growing prevalence of chronic and genetic conditions. The urgent need for accurate and timely diagnostics, especially highlighted during the COVID-19 pandemic, has accelerated interest and investment in CRISPR-based tools. Additionally, collaborations between biotech firms, academic institutions, and public health agencies are fueling innovation and expediting commercialization.
The market holds significant opportunities, particularly in point-of-care diagnostics, personalized medicine, and early detection of rare or antimicrobial-resistant diseases. Recent partnerships, such as those between Illumina and the Broad Institute and between CRISPRbits and Molbio Diagnostics, underscore the growing momentum and commercial interest in this space.
Executive Summary
For more details on this report – Request for Sample
CRISPR-Based Diagnostics Market Dynamics: Drivers & Restraints
Rising prevalence and incidence of infectious diseases are expected to drive the CRISPR-based diagnostics market
The development of rapid and sensitive diagnostic tools is critical for effective patient management and controlling the spread of infectious diseases. As the global burden of disease continues to rise, there is an urgent demand for faster, more accessible diagnostic methods. For instance, in 2024, the CDC reported at least 35 million flu cases, resulting in 400,000 hospitalizations and 25,000 deaths in a single season.
While current diagnostics are highly specific and sensitive, they often rely on time-consuming processes, expensive laboratory infrastructure, and trained personnel, limiting their usability in resource-limited settings, large-scale screenings, and during outbreaks.
Dengue threatens nearly half of the global population, with an estimated 100–400 million infections occurring each year. Dengue fever further emphasizes the importance of rapid diagnostics. Timely and accurate diagnosis is crucial for managing individual cases and preventing widespread transmission.
CRISPR-based diagnostic technologies are emerging as transformative tools. These systems offer rapid, highly specific, and sensitive detection, allowing healthcare providers to differentiate dengue from other similar illnesses and make prompt, informed decisions. Consequently, the increasing demand for efficient and accessible diagnostic solutions amid growing infectious disease threats is expected to drive significant growth in the CRISPR-based diagnostics market.
High costs of CRISPR-based diagnostic tools and systems
Developing, manufacturing, and scaling CRISPR diagnostic tools involve substantial investment in specialized reagents, advanced equipment, and skilled personnel. These factors drive up the overall cost of each test, making it less accessible, particularly in low- and middle-income countries or in resource-limited healthcare settings where affordability is a critical factor.
Moreover, although CRISPR technologies are often promoted as simpler alternatives to conventional molecular diagnostics, the initial setup costs, including the need for controlled environments and supporting infrastructure, can be a barrier to widespread adoption.
CRISPR-Based Diagnostics Market Segment Analysis
The global CRISPR-based diagnostics market is segmented based on technique, product, application, end user, and region.
Technique:
The recombinase polymerase amplification (RPA) segment is expected to hold 38.6% of the global CRISPR-based diagnostics market
The recombinase polymerase amplification (RPA) segment is poised to dominate the CRISPR-based diagnostics market due to its compatibility with point-of-care applications and its cost-effectiveness. RPA, an isothermal amplification technique, enables rapid nucleic acid amplification without the need for complex thermal cycling equipment, making it particularly suitable for resource-limited settings and large-scale screenings.
When combined with CRISPR-based systems, such as CRISPR/Cas12a, RPA enhances the sensitivity and specificity of diagnostics, allowing for the detection of low-abundance targets with high precision. Recent advancements underscore the growing adoption of RPA in CRISPR-based diagnostics. For instance, a study developed a CRISPR/Cas12a platform coupled with RPA for the rapid detection of antimicrobial-resistant genes in carbapenem-resistant Enterobacterales, demonstrating its potential for infectious disease diagnostics.
CRISPR-Based Diagnostics Market Geographical Analysis
North America is expected to hold 36.9% of the global CRISPR-based diagnostics market
In North America, the escalating prevalence of chronic diseases, genetic disorders, and infectious diseases has intensified the demand for innovative and efficient diagnostic methods. For instance, the Centers for Disease Control and Prevention (CDC) reported that in 2023, the United States experienced a significant rise in tuberculosis (TB) cases, with a total of 9,615 cases, a 16% increase from 2022 and the highest number reported in a decade. This surge underscores the urgent need for rapid and accurate diagnostic tools to manage and control such infectious diseases.
In response to this growing demand, there has been a notable increase in collaborations among government agencies, commercial enterprises, and academic institutions across the region. These partnerships are fostering innovation and accelerating the translation of research into practical applications.
For instance, in August 2024, Illumina announced a strategic partnership with the Broad Institute to develop new gene sequencing kits that enable large-scale sequencing using an innovative approach based on the Nobel Prize-winning CRISPR technology. In a separate collaboration involving the Broad Institute and Harvard University, Illumina will also focus on single-cell research sequencing. This initiative will utilize advanced technology from Fluent BioSciences to conduct cutting-edge experiments aimed at enhancing the precision and scalability of genomic research.
These technologies are particularly appealing to healthcare professionals seeking efficient solutions to combat the spread of infectious diseases. Collectively, the increasing incidence of diseases, coupled with strategic collaborations and the increased demand for rapid diagnostic solutions, is expected to drive significant growth in the CRISPR-based diagnostics market in North America during the forecast period.
CRISPR-Based Diagnostics Market Top Companies
The top companies in the CRISPR-based diagnostics market include OraSure Technologies, Inc., Mammoth Biosciences, Inc., Integrated DNA Technologies, GenScript, among others.
Key Developments
In January 2025, CrisprBits Private Limited inaugurated a state-of-the-art laboratory dedicated to advancing CRISPR-based diagnostic solutions. The new facility is focused on developing innovative diagnostics for rare diseases, including hospital-acquired infections and antimicrobial resistance, leveraging the precision and power of CRISPR gene editing technology to address critical gaps in current diagnostic capabilities.
In August 2023, CrisprBits announced a strategic collaboration with Molbio Diagnostics to advance the integration of CRISPR into Point-of-Care (POC) testing. This partnership is designed to leverage the combined strengths and expertise of both companies to identify and capitalize on emerging market opportunities. By uniting CrisprBits’ cutting-edge CRISPR capabilities with Molbio’s proven diagnostic platforms, the collaboration aims to accelerate the development of rapid, accurate, and accessible POC diagnostic solutions.
Market Scope
Metrics | Details | |
CAGR | 16.7% | |
Market Size Available for Years | 2022-2033 | |
Estimation Forecast Period | 2025-2033 | |
Revenue Units | Value (US$ Bn) | |
Segments Covered | Technique | Recombinase Polymerase Amplification (RPA), Loop-mediated Isothermal Amplification (LAMP), Others |
Product | Kits, Buffer Solutions, Software, Others | |
Application | Infectious Diseases, Non-Infectious Diseases | |
End User | Hospitals, Diagnostic Centers, Biotechnology Companies, Academic and Research Institutes, Others | |
Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
The global CRISPR-based diagnostics market report delivers a detailed analysis with 57 key tables, more than 46 visually impactful figures, and 168 pages of expert insights, providing a complete view of the market landscape.

