Market Size
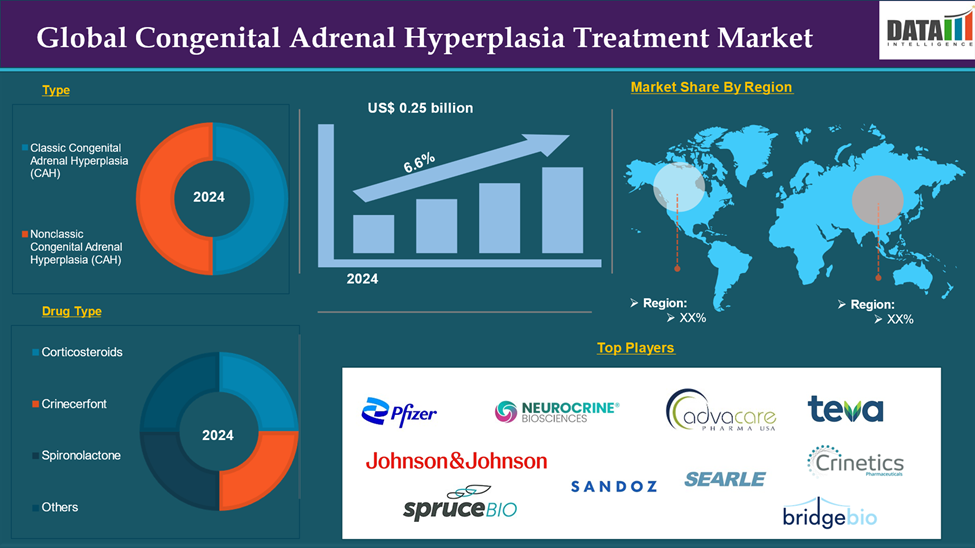
The Global Congenital Adrenal Hyperplasia Treatment Market reached US$ 0.25 billion in 2024 and is expected to reach US$ 0.41 billion by 2033, growing at a CAGR of 6.6% during the forecast period 2025-2033.
Congenital adrenal hyperplasia (CAH) is an autosomal recessive disorder caused by a mutation in the gene responsible for producing an enzyme essential for adrenal hormone synthesis. In individuals with CAH, the inability to produce cortisol disrupts the normal feedback loop of the hypothalamic-pituitary-adrenal (HPA) axis. This leads to excessive secretion of adrenocorticotropic hormone (ACTH), adrenal gland hyperplasia, and elevated adrenal androgen levels.
As a result, CAH patients may experience premature puberty, impaired fertility, hirsutism, acne, adrenal rest tumors, and a reduced quality of life. Female patients may also develop virilized genitalia and experience menstrual irregularities. Currently, the only available treatment to suppress excess androgen production involves administering supraphysiologic doses of glucocorticoids. However, long-term GC therapy carries significant risks, including diabetes, cardiovascular disease, stunted growth, osteoporosis, skin thinning, gastrointestinal complications, and a reduced lifespan.
Executive Summary
For more details on this report – Request for Sample
Market Dynamics: Drivers & Restraints
Investments in Research and Development
The investments in research and development are significantly driving the growth of the global congenital adrenal hyperplasia treatment market and are expected to drive throughout the market forecast period. The congenital adrenal hyperplasia (CAH) treatment market is experiencing notable progress in medical research, with the introduction of innovative therapies like Crinecerfont, SPR001, Chronocort, and AAV BBP-631. These developments aim to enhance treatment effectiveness while alleviating the dependency on glucocorticoids and mineralocorticoids, addressing both therapeutic gaps and long-term complications associated with traditional treatment approaches.
Research and development (R&D) are pivotal to the growth and evolution of the global congenital adrenal hyperplasia (CAH) treatment market. Significant R&D investments are helping to create new treatment options beyond traditional glucocorticoid therapy, such as gene therapies, ACTH receptor antagonists, and other targeted therapies. These advancements aim to address the root cause of CAH rather than just managing symptoms, offering the potential for more effective and long-term solutions.
R&D funding allows for deeper research into the genetic and biochemical mechanisms of CAH. A better understanding of these processes can lead to the development of therapies that more precisely target the disease, reducing side effects associated with current treatments. Ongoing clinical trials funded by R&D investments are leading to new drug approvals from regulatory bodies like the FDA, which is opening up opportunities for novel treatments in the market.
Additionally, key players in the industry are more focused on the research and developments that would drive this congenital adrenal hyperplasia treatment market growth. For instance, in December 2024, Spruce Biosciences, Inc. reported topline results from its CAHmelia-204 study of tildacerfont in adults with congenital adrenal hyperplasia (CAH) and its CAHptain-205 study in both adult and pediatric CAH patients.
Also, in June 2024, Crinetics Pharmaceuticals, Inc. announced initial findings from the development program of its second clinical candidate, atumelnant (CRN04894), a once-daily oral adrenocorticotropic hormone (ACTH) receptor antagonist. The results, presented at the Endocrine Society’s annual meeting (ENDO2024), include preliminary data from a Phase 1b/2a open-label study in patients with ACTH-dependent Cushing’s syndrome (ADCS) conducted in collaboration with the National Institutes of Health (NIH). All these factors demand the global congenital adrenal hyperplasia treatment market.
Moreover, the rising demand for emerging non-steroidal therapies contributes to the global congenital adrenal hyperplasia treatment market expansion.
High Cost of Treatment
The high cost of treatment may hinder the growth of the global congenital adrenal hyperplasia treatment market. The treatment of congenital adrenal hyperplasia (CAH), particularly the classic form of the disease, often involves lifelong management with glucocorticoid (GC) therapy and mineralocorticoids, both of which can be expensive.
Additionally, frequent monitoring and adjustments in doses based on changes in the patient's condition further increase healthcare costs. The high direct costs of medical care are compounded by indirect costs, such as reduced productivity, increased healthcare visits, and long-term management of related complications (e.g., osteoporosis, diabetes, cardiovascular diseases), all of which contribute to the overall financial burden.
Market Segment Analysis
The global congenital adrenal hyperplasia treatment market is segmented based on type, drug type, route of administration, distribution channel, and region.
Drug Type:
The hydrocortisone in the drug type segment is expected to dominate with the highest market share.
Hydrocortisone plays a central role in the treatment of congenital adrenal hyperplasia (CAH), a rare genetic disorder that affects the adrenal glands. The global CAH treatment market is primarily segmented by the types of therapies used, including traditional glucocorticoids like hydrocortisone, newer biologics, and emerging therapies. Hydrocortisone remains one of the most commonly used treatments for CAH, specifically in its modified-release form, which helps replicate the natural circadian rhythm of cortisol production.
The conventional formulation of hydrocortisone is commonly used in CAH patients to manage adrenal insufficiency and prevent adrenal crisis. However, it requires multiple daily doses, which can be challenging for patients. Modified formulations, such as Chronocort (developed by Diurnal Ltd.), are designed to mimic natural cortisol secretion and require fewer doses per day. These formulations help reduce the frequency of administration, improve treatment adherence, and offer more physiological cortisol levels throughout the day.
Hydrocortisone is often used in combination with other therapies, such as fludrocortisone, to manage electrolyte imbalances in CAH patients. It may also be combined with emerging treatments like ACTH receptor antagonists or gene therapies to provide more comprehensive management.
Market Geographical Analysis
North America is expected to hold a significant position in the global congenital adrenal hyperplasia treatment market.
North America holds a substantial position in the global congenital adrenal hyperplasia treatment market and is expected to hold most of the market share in the forecast period. The key factors contributing to the region’s dominance include advancements in medical care, the availability of new therapies, and high healthcare access.
Additionally, in this region, a major number of key players are present, with well-advanced healthcare infrastructure, product launches, approvals, and a rising number of clinical trials. For instance, in December 2024, the U.S. Food and Drug Administration (FDA) approved crinecerfont (Crenessity) as a treatment for classic congenital adrenal hyperplasia (CAH). This marks the first new therapeutic option in 70 years for both adults and children aged 4 and older affected by this rare, lifelong condition.
Also, in May 2024, Neurocrine Biosciences, Inc. presented baseline data from the CAHtalyst Phase 3 studies of crinecerfont in both adult and pediatric patients with congenital adrenal hyperplasia (CAH). Additionally, they shared data from a Phase 2 clinical study (CHAMPAIN) involving modified-release hydrocortisone (Chronocort) in participants with primary adrenal insufficiency, as well as results from a Phase 3 extension study in CAH patients. Thus, the above factors are consolidating the region's position as a dominant force in the global congenital adrenal hyperplasia treatment market.
Asia Pacific is growing at the fastest pace in the global congenital adrenal hyperplasia treatment market share
Asia Pacific holds the fastest pace in the global congenital adrenal hyperplasia treatment market and is expected to hold most of the market share. The prevalence of CAH in the Asia-Pacific is higher than in other regions, particularly among certain populations, which increases the demand for targeted treatments. Countries with large populations, such as India and China, are seeing rising numbers of CAH cases, further expanding the patient pool and the need for effective treatments.
Awareness of CAH is steadily growing across the Asia-Pacific region, especially as more healthcare providers are recognizing the importance of early diagnosis through newborn screening programs. This has led to earlier identification and treatment of the disease, helping to improve patient outcomes and driving demand for treatment options. Countries like Japan, South Korea, and Australia have made strides in genetic testing and screening programs, leading to better detection rates of CAH, which in turn boosts the market for CAH treatments.
Developed countries like Japan, Australia, and South Korea have advanced healthcare systems with widespread access to specialty care. These regions have well-established healthcare networks and often have access to cutting-edge treatments such as modified-release hydrocortisone and newer biologics. In emerging markets like India and China, improvements in healthcare infrastructure and access to modern medical facilities have made it easier for patients to receive timely diagnosis and treatment, fostering market growth.
Emerging therapies, such as modified-release hydrocortisone, gene therapies, and ACTH receptor antagonists, are gaining traction in the Asia-Pacific market. As global pharmaceutical companies continue to expand their reach in this region, more innovative treatments are becoming available to patients with CAH.
Additionally, key players' strategies, such as partnerships and collaborations, would drive this global congenital adrenal hyperplasia treatment market growth. For instance, in January 2023, Spruce Biosciences and Kaken Pharmaceutical announced a strategic partnership and exclusive licensing agreement to develop and commercialize Tildacerfont for the treatment of congenital adrenal hyperplasia (CAH) in Japan. Under the terms of the agreement, Kaken will have the first right of negotiation to extend the agreement to include China (including Hong Kong, Taiwan, and Macau), South Korea, and other specified Southeast Asian (ASEAN) countries. Additionally, Kaken will be responsible for securing and maintaining the necessary regulatory approvals to market and sell Tildacerfont in Japan.
Also, in January 2021, Citrine Medicine announced a strategic partnership with Diurnal Group plc to bring Alkindi to China for the treatment of pediatric congenital adrenal hyperplasia (CAH). The partnership involves a licensing agreement, allowing Citrine Medicine to commercialize Alkindi® in the Chinese market. Alkindi is a medication used to manage CAH in children, specifically designed to provide more precise dosing compared to conventional treatments. Thus, the above factors are consolidating the region's position as the fastest-growing force in the global congenital adrenal hyperplasia treatment market.
Major Global Players
The major global players in the congenital adrenal hyperplasia treatment market include Neurocrine Biosciences, Inc., Pfizer Inc., Johnson & Johnson Services, Inc., AdvaCare Pharma, Teva Pharmaceuticals USA, Inc., Sandoz, THE SEARLE COMPANY LTD., Spruce Biosciences, Inc., Crinetics Pharmaceuticals, Inc., BridgeBio Inc., and H. Lundbeck A/S among others.
Key Developments
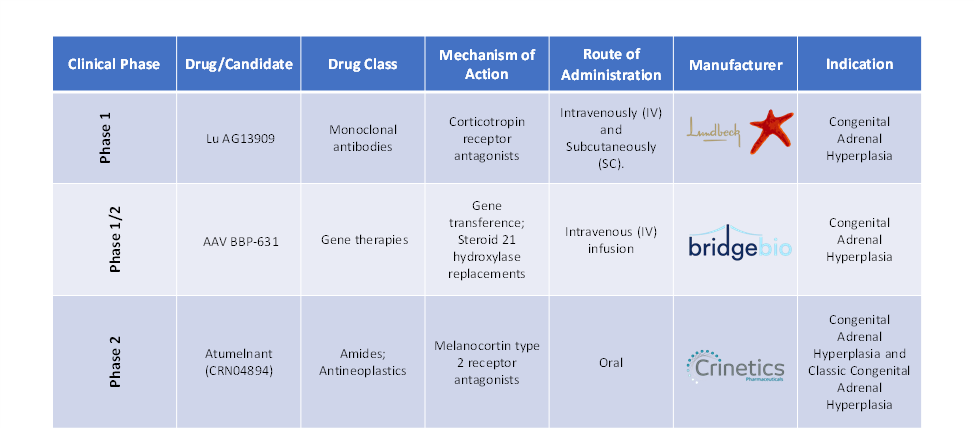
- In January 2025, Crinetics Pharmaceuticals, Inc. reported positive topline results from a Phase 2 clinical trial evaluating atumelnant, an investigational drug for classic congenital adrenal hyperplasia (CAH) and ACTH-dependent Cushing’s syndrome.
- In September 2024, BridgeBio Pharma, Inc. announced topline results from the Phase 1/2 ADventure study, an open-label trial evaluating BBP-631, the company’s investigational adeno-associated virus (AAV) 5 gene therapy for the treatment of congenital adrenal hyperplasia (CAH).
- In May 2024, Spruce Biosciences will provide an update on the company's financial performance for the first quarter of 2024, along with key corporate and clinical developments. The analysis of data from the CAHmelia-203 study (a clinical trial evaluating tildacerfont in adults with congenital adrenal hyperplasia [CAH]) found that patients' response to tildacerfont correlated with their baseline glucocorticoid (GC) dose and drug compliance.
| Metrics | Details | |
| CAGR | 6.6% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Type | Classic Congenital Adrenal Hyperplasia (CAH), Nonclassic Congenital Adrenal Hyperplasia (CAH) |
| Drug Type | Corticosteroids, Crinecerfont, Spironolactone, Others | |
| Route of Administration | Oral, Injectable | |
| Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials and product pipelines and forecasts upcoming advancements in medical devices and pharmaceuticals.
- Product Performance & Market Positioning: Analyze product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: This covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyze competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient product delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global congenital adrenal hyperplasia treatment market report delivers a detailed analysis with 60+ key tables, more than 50 visually impactful figures, and 176 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2024
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.
