Overview
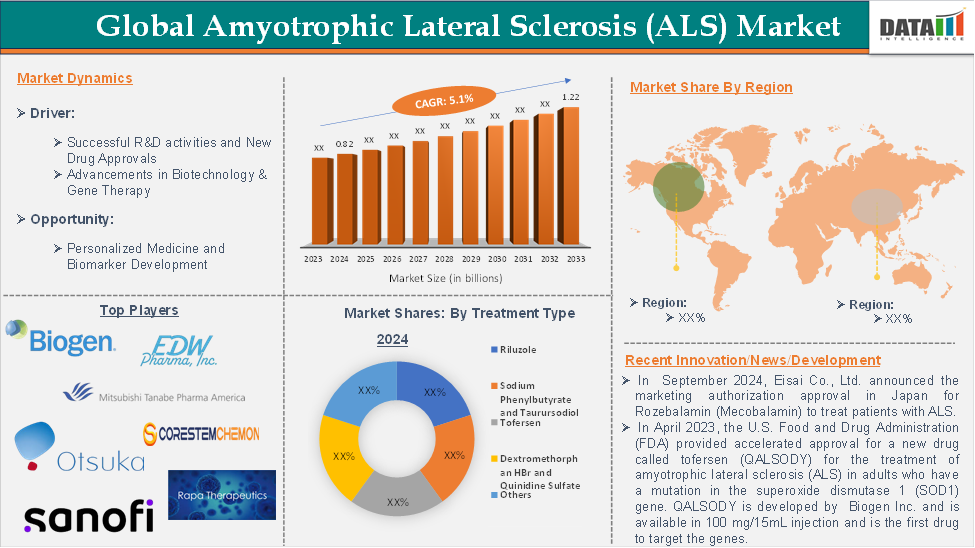
The global amyotrophic lateral sclerosis (ALS) market reached US$ 0.82 billion in 2024 and is expected to reach US$ 1.22 billion by 2033, growing at a CAGR of 5.1 % during the forecast period of 2025-2033.
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, is a progressive neurodegenerative disorder that affects the motor neurons. These neurons control the body’s muscle movement and respiration. A person affected with ALS experiences progressive loss of motor function, muscle weakness, loss of movement, and later the ability to breathe. The causes of ALS are unknown, but research efforts have confirmed the role of genetics to be the main cause in certain cohorts.
The existing treatment modalities of ALS majorly focus on slowing down the disease progression and include drugs like Riluzole, Edaravone, Tofersen, etc. In addition to pharmacotherapy, multidisciplinary care, including physical therapy, respiratory support, and nutritional interventions, is crucial for extending survival and enhancing patient well-being.
Executive Summary
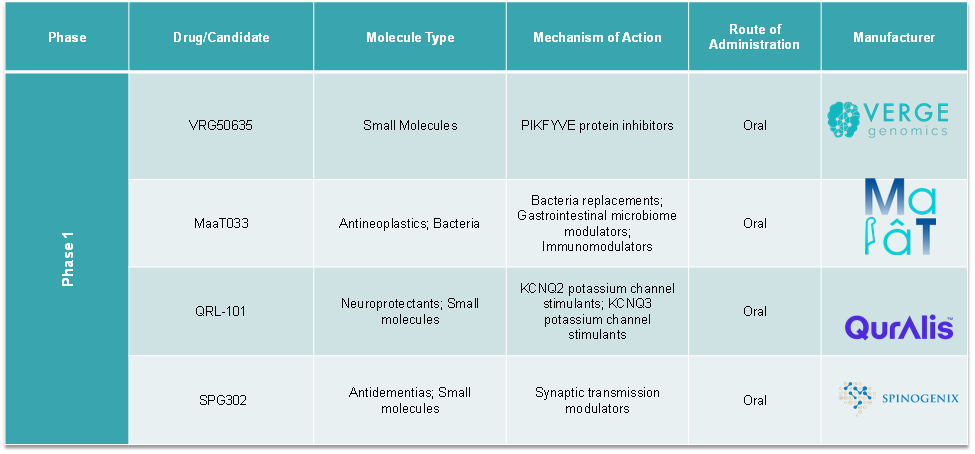
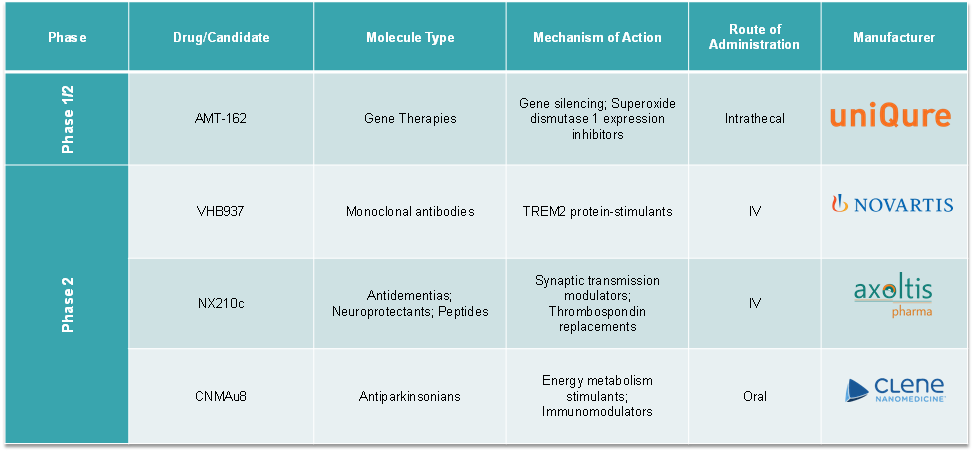
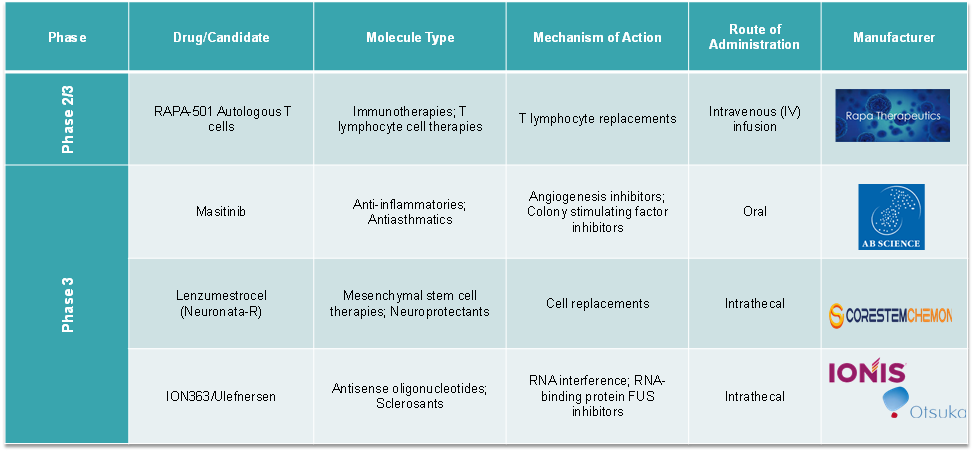
Pipeline Analysis
Market Dynamics: Drivers & Restraints
Successful R&D Activities and New Drug Approvals
ALS is fatal, and only a handful of drugs are currently approved for slowing down the disease’s progression and improving the patient’s quality of life. In recent times, several drugs have received regulatory nod for marketing authorization in various regions across the globe.
For instance, in April 2023, the U.S. Food and Drug Administration (FDA) provided accelerated approval for a new drug called tofersen (QALSODY), for the treatment of amyotrophic lateral sclerosis (ALS) in adults who have a mutation in the superoxide dismutase 1 (SOD1) gene. QALSODY is developed by Biogen Inc. is available in 100 mg/15mL injection and is the first drug to target the genes.
QALSODY received marketing authorization in the European Union (EU) in May 2024 and is the second drug after riluzole authorized for use in the EU for ALS.
Moreover, in September 2024, Eisai Co., Ltd. announced the marketing authorization approval in Japan for Rozebalamin (Mecobalamin) to treat patients with ALS. These recent drug approvals widen the treatment options for ALS patients and improve the overall treatment rate, driving the market growth.
Drug Discontinuation
The treatment options for ALS are very limited, with only a handful of drugs approved and available on the market. Any discrepancy in the supply chain of these drugs, or product discontinuation may impact the treatment adherence by patients and impact the overall market growth.
A notable example is the recent drug discontinuation by Mitsubishi Tanabe Pharma America, Inc. (MTPA). Mitsubishi Tanabe is commercializing EXSERVAN, which is a riluzole oral film formulation in the U.S. Market. However, in July 2024, the company decided to discontinue Exservan.
Moreover, MTPA also announced the discontinuation of intravenous RADICAVA (edaravone) in October 2024. The drug was first approved in 2017 by the U.S. FDA and will not be available to patients from April 2025. In addition, one of the novel drugs developed for ALS, i.e., RELYVRIO/ALBRIOZA by AMYLYX Pharmaceuticals, was also withdrawn from the market in 2024. These drug withdrawals can significantly hinder the treatment rate and the market supply chain.
Segment Analysis
The global amyotrophic lateral sclerosis (ALS) market is segmented based on disease type, treatment type, distribution channel, and region.
Treatment Type:
The riluzole segment in treatment type is expected to dominate the global amyotrophic lateral sclerosis (ALS) market with the highest market share
Riluzole was the first drug to be indicated for ALS, and its long-standing presence has earned it widespread clinical adoption and trust among healthcare professionals. It remains the most commonly prescribed treatment for ALS globally. This leadership is driven by its proven efficacy in extending patient survival and delaying the progression of the disease, especially in its earlier stages.
Riluzole functions by inhibiting the release of glutamate, a neurotransmitter that, in excessive amounts, is neurotoxic and contributes to the degeneration of motor neurons, a hallmark of ALS. By regulating this excitotoxicity, Riluzole helps reduce neuronal damage, thereby modestly prolonging life expectancy and delaying the need for invasive respiratory support in ALS patients. Although it is not curative and its benefits are limited to slowing progression rather than halting or reversing it, its role as a foundational treatment in ALS therapy remains strong.
Another key factor contributing to Riluzole's dominance is its global accessibility and the availability of generic formulations, which make it cost-effective and widely distributed in both developed and emerging markets. It is available in multiple forms, including tablets and oral suspensions, the latter catering to ALS patients who often develop difficulties with swallowing. These formulations have expanded their usability across different stages of disease severity.
Despite the advent of newer treatments such as Edaravone, Tofersen, and experimental gene therapies, Riluzole continues to play a vital role in ALS treatment protocols. It is frequently used in combination with other drugs to create a multi-pronged approach to disease management. These factors have solidified the segment's position in the global amyotrophic lateral sclerosis (ALS) market.
Geographical Analysis
North America is expected to hold a significant position in the global amyotrophic lateral sclerosis (ALS) market with the highest market share
North America dominates the ALS market due to its advanced healthcare infrastructure and higher prevalence of the condition. For instance, as per the epidemiological study published in the Taylor and Francis Journal, the total prevalence cases of ALS in the U.S. in 2022 was 32,893. By 2030 these cases are expected to reach 36,308 with a rise of approximately 10%.
North America, particularly the United States and Canada, possesses well-developed healthcare systems with robust infrastructure, including hospitals, specialty clinics, research institutions, and rehabilitation centers. This infrastructure facilitates the timely diagnosis, treatment, and management of ALS patients. Moreover, advanced mobility aids, communication devices, respiratory support systems, and other assistive technologies are readily available in the region.
In addition, any novel drug discovered for ALS first gets launched in the U.S. market due to higher demand. Hence, the patient population gets first access to these interventions. Moreover, manufacturers tend to generate most of their revenue from the region due to higher costs and higher demand. Thus, the above factors are consolidating the region's position as a dominant force in the global amyotrophic lateral sclerosis (ALS) market.
Competitive Landscape
The major global players in the amyotrophic lateral sclerosis (ALS) market include BIOGEN, Sanofi, Otsuka Holdings Co., Ltd., Mitsubishi Tanabe Pharma, EDW Pharma, Inc., BrainStorm Cell Therapeutics, Eisai Co., Ltd., Rappta Therapeutics, AB Science, and CorestemChemon Inc., among others.
Key Developments
- In September 2024, Eisai Co., Ltd. announced the marketing authorization approval in Japan for Rozebalamin (Mecobalamin) to treat patients with ALS.
- In August 2023, the U.S. Food and Drug Administration (FDA) provided accelerated approval for a new drug called tofersen (QALSODY) for the treatment of amyotrophic lateral sclerosis (ALS) in adults who have a mutation in the superoxide dismutase 1 (SOD1) gene. QALSODY is developed by Biogen Inc. and is available in 100 mg/15mL injection and is the first drug to target the genes.
| Metrics | Details | |
| CAGR | 5.1% | |
| Market Size Available for Years | 2022-2033 | |
| Estimation Forecast Period | 2025-2033 | |
| Revenue Units | Value (US$ Bn) | |
| Segments Covered | Disease Type | Sporadic ALS, Familial ALS (FALS) |
| Treatment Type | Riluzole, Edaravone, Sodium Phenylbutyrate and Taurursodiol, Tofersen, Dextromethorphan HBr and Quinidine Sulfate, Others | |
| Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies | |
| Regions Covered | North America, Europe, Asia-Pacific, South America, and the Middle East & Africa | |
Why Purchase the Report?
- Pipeline & Innovations: Reviews ongoing clinical trials and product pipelines and forecasts upcoming advancements in medical devices and pharmaceuticals.
- Product Performance & Market Positioning: Analyzed product performance, market positioning, and growth potential to optimize strategies.
- Real-World Evidence: Integrates patient feedback and data into product development for improved outcomes.
- Physician Preferences & Health System Impact: Examines healthcare provider behaviors and the impact of health system mergers on adoption strategies.
- Market Updates & Industry Changes: This covers recent regulatory changes, new policies, and emerging technologies.
- Competitive Strategies: Analyze competitor strategies, market share, and emerging players.
- Pricing & Market Access: Reviews pricing models, reimbursement trends, and market access strategies.
- Market Entry & Expansion: Identifies optimal strategies for entering new markets and partnerships.
- Regional Growth & Investment: Highlights high-growth regions and investment opportunities.
- Supply Chain Optimization: Assesses supply chain risks and distribution strategies for efficient product delivery.
- Sustainability & Regulatory Impact: Focuses on eco-friendly practices and evolving regulations in healthcare.
- Post-market Surveillance: Uses post-market data to enhance product safety and access.
- Pharmacoeconomics & Value-Based Pricing: Analyzes the shift to value-based pricing and data-driven decision-making in R&D.
The global amyotrophic lateral sclerosis (ALS) market report delivers a detailed analysis with 62 key tables, more than 53 visually impactful figures, and 176 pages of expert insights, providing a complete view of the market landscape.
Target Audience 2024
- Manufacturers: Pharmaceutical, Medical Device, Biotech Companies, Contract Manufacturers, Distributors, Hospitals.
- Regulatory & Policy: Compliance Officers, Government, Health Economists, Market Access Specialists.
- Technology & Innovation: AI/Robotics Providers, R&D Professionals, Clinical Trial Managers, Pharmacovigilance Experts.
- Investors: Healthcare Investors, Venture Fund Investors, Pharma Marketing & Sales.
- Consulting & Advisory: Healthcare Consultants, Industry Associations, Analysts.
- Supply Chain: Distribution and Supply Chain Managers.
- Consumers & Advocacy: Patients, Advocacy Groups, Insurance Companies.
- Academic & Research: Academic Institutions.

