For many years, there was no treatment for Friedreich’s Ataxia, only care to manage symptoms. But recently, there’s been real progress, with the first drug approved, gene therapy trials underway, and many biotech companies working to change things.
The First FDA-Approved Drug for Friedreich’s Ataxia
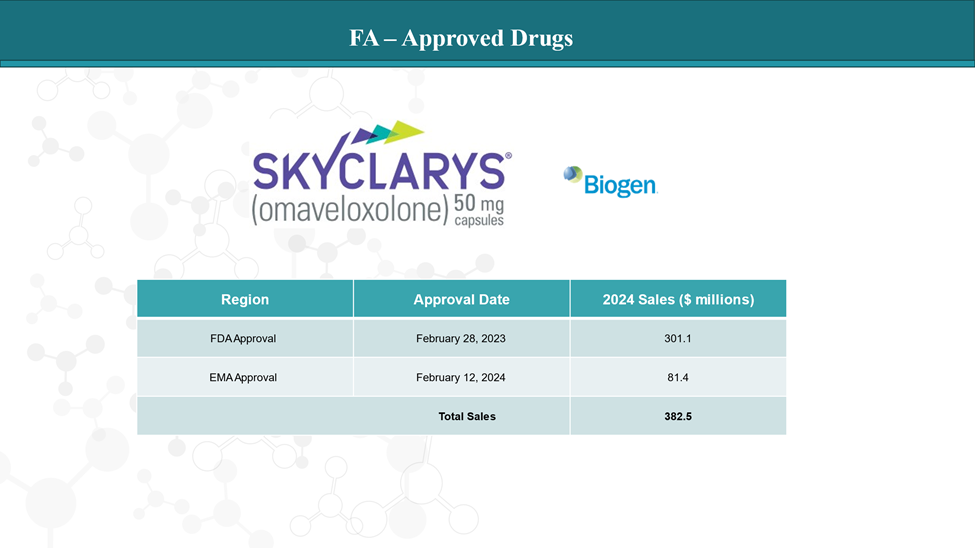
In February 2023, the U.S. FDA approved Skyclarys (omaveloxolone), developed by Reata Pharmaceuticals (now a part of Biogen), marking a historic milestone as the first-ever approved treatment for FA.
How Skyclarys Works
Skyclarys is a Nrf2 activator, which helps combat oxidative stress—a key contributor to the degeneration seen in FA. In the MOXIe trial, patients taking omaveloxolone showed improvements in neurological function and slowed progression of the disease, particularly in mobility and coordination.
It’s not a cure, but it’s a crucial first step—one that validates FA as a druggable disease and proves that therapeutic intervention can make a real difference.
The Pipeline: Who’s Competing?
With Skyclarys paving the way, the FA landscape is heating up with biotech and pharmaceutical companies racing to bring better, more durable therapies to market. Here's a look at some of the key players:
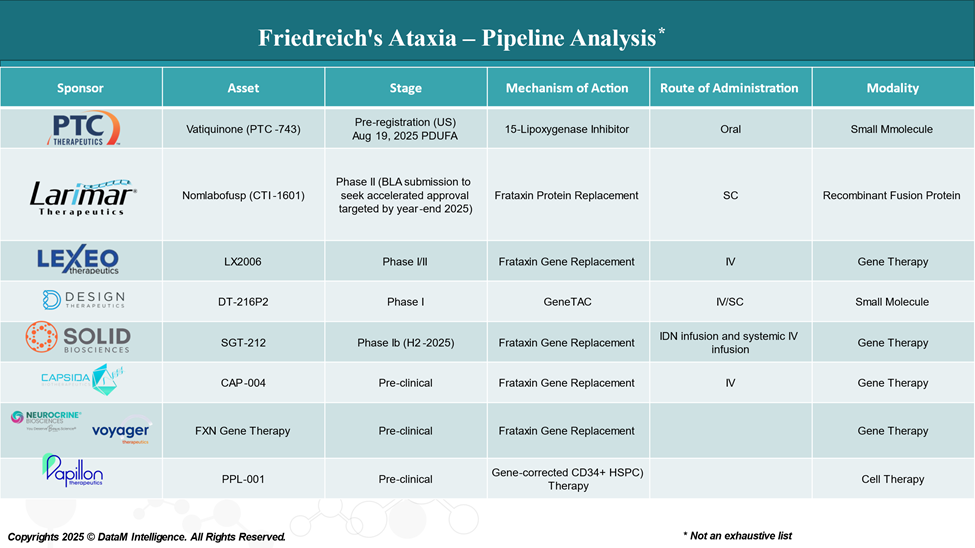
Gene Therapy Leaders
- Lexeo Therapeutics - LX2006
- Focus: Cardiac-directed AAV gene therapy for FA cardiomyopathy
- Status: Early-phase clinical trials
- Goal: Deliver a functioning FXN gene directly to heart cells to prevent or reverse damage.
- Voyager Therapeutics/Neurocrine Biosciences
- Exploring FXN gene therapy platforms that target the central nervous system and spinal cord, potentially delivering FXN systemically.
- Solid Biosciences - SGT-212
- Developing gene therapies that deliver functional frataxin using AAV9 vectors, aiming for whole-body correction, including neurological and cardiac tissue.
Small Molecule & Protein Replacement Players
- PTC Therapeutics is investigating Vatiquinone (15-Lipoxygenase Inhibitor), a small molecule that targets mitochondrial function and inflammation. FDA expected approval date is August 19, 2025.
- Larimar Therapeutics is working on Nomlabofusp (CTI-1601), a frataxin protein replacement therapy, administered via injection to increase frataxin levels in cells.
These companies represent a wide range of approaches—from fixing the genetic root to restoring protein function and mitigating symptoms.
Why Gene Therapy Matters in FA?
Gene therapy holds curative potential. Since Friedreich’s Ataxia stems from a single-gene mutation (GAA repeat expansion in the FXN gene), it is an ideal target for gene replacement or editing.
A successful gene therapy could:
- Deliver a healthy copy of the FXN gene
- Restore normal levels of frataxin protein
- Potentially halt or even reverse disease progression
- Require one-time administration (depending on vector durability)
Beyond the Breakthrough: What the Future Holds for FA
Gene therapies are still in early stages, and long-term safety and efficacy data are being collected.
At the same time, there’s increasing investment and patient advocacy, fueled by organizations like FARA (Friedreich’s Ataxia Research Alliance), helping accelerate progress across the board.
Final Thoughts
There are now better treatment options for Friedreich’s Ataxia. The first drug has been approved, and new gene therapies are being developed, giving real hope to patients.
As more companies get involved, the race to find a treatment for Friedreich’s Ataxia is heating up. For patients, this competition brings hope that the disease will be treatable and no longer a lifelong struggle.

