Fabry disease, a rare inherited disorder, has long posed major challenges for patients and physicians. Caused by mutations in the GLA gene leading to deficiency in the enzyme alpha-galactosidase A, it results in the accumulation of a fatty substance called Gb3 (globotriaosylceramide) in the body’s tissues.
Traditionally, Fabry disease has been managed with enzyme replacement therapy (ERT)—biweekly intravenous infusions that replace the missing enzyme. While effective in slowing disease progression, ERT comes with challenges: it’s time-consuming, expensive, and sometimes causes immune reactions.
Then came chaperone therapy, in the form of an oral drug called migalastat, designed for patients with specific genetic mutations. This was a breakthrough in convenience, but its use is limited to a subset of patients.
Now, emerging therapies are changing the game:
A New Era of Emerging Fabry Therapies
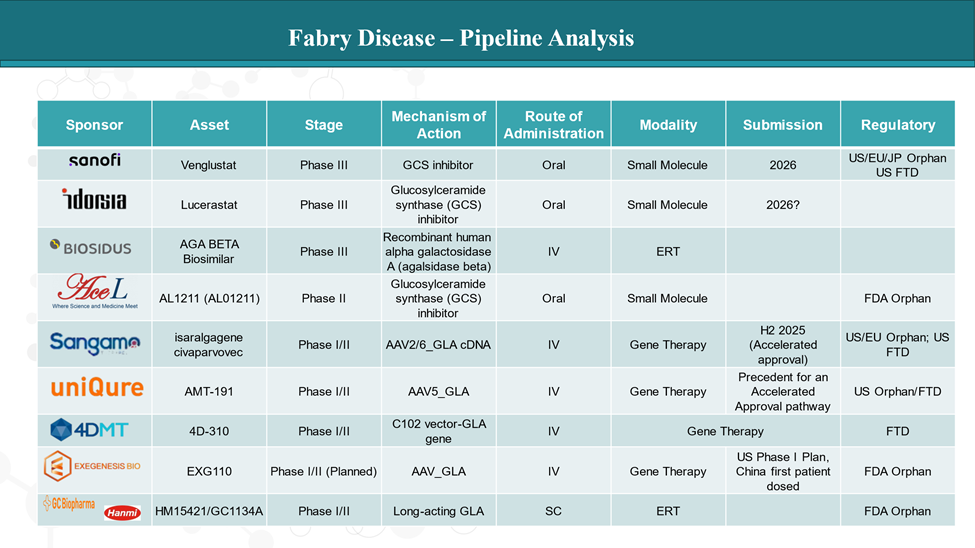
The next wave of Fabry therapies includes gene therapy, oral small molecules, biosimilars, and improved enzyme formulations.
Gene Therapy: Aiming for a One-Time Cure
- Isaralgagene civaparvovec (Sangamo Therapeutics)
This gene therapy uses an AAV vector to deliver a healthy copy of the GLA gene to liver cells. The goal is long-term enzyme production from a single infusion, reducing or even eliminating the need for regular treatment. - AMT-191 (uniQure)
A liver-directed gene therapy with enhanced design for high sustained enzyme expression. Early data aim to show long-term Gb3 reduction and systemic impact. - 4D-310 (4D Molecular Therapeutics)
A gene therapy designed to specifically target the heart, a major organ impacted in Fabry disease, using a cardiac-tropic viral vector to directly deliver the GLA gene. - EXG110 (Exegenesis Bio)
Another promising AAV-based candidate still in early development, aimed at correcting the enzyme deficiency with potentially a one-time treatment.
Oral and Small Molecule Therapies: Convenient and Targeted
- Venglustat (Sanofi)
A substrate reduction therapy (SRT) that reduces the buildup of Gb3 by inhibiting its formation. Taken orally, this could serve as an alternative or adjunct to enzyme therapies. - Lucerastat (Idorsia)
Another SRT taken by mouth, Lucerastat targets the underlying lipid accumulation in all Fabry patients, regardless of their specific mutation. - AL1211 (AceLink Therapeutics)
A new oral glucosylceramide synthase inhibitor is designed to work in all Fabry patients. Aims for high potency with fewer off-target effects.
Next-Generation and Biosimilar Enzymes
- AGA BETA Biosimilar (Bio Sidus SA)
A biosimilar version of agalsidase beta (Fabrazyme), which could reduce treatment costs while maintaining similar efficacy and safety. - HM15421/GC1134A (Hanmi Pharmaceutical/GC Biopharma)
A long-acting enzyme therapy with a proprietary half-life extension technology. Designed for less frequent dosing and improved patient adherence.
What This Means for Patients
The emerging therapies offer improvements across several key areas:
| Area | Current Limitations | New Therapeutic Potential |
| Convenience | Biweekly IV infusions | Oral pills or one-time gene therapies |
| Efficacy | Partial Gb3 clearance, limited CNS penetration | More complete, targeted organ effects (e.g., heart, brain) |
| Safety | Immune reactions, infusion-related side effects | Reduced immunogenicity, better tolerability |
| Cost & Access | High lifetime costs with ERT | Biosimilars and gene therapy could reduce the total cost |
| Personalization | Limited by mutation-specific drugs | Broad applicability across Fabry mutations (e.g., Lucerastat, gene therapies) |
Looking Ahead: A Brighter Future for Fabry Patients
These emerging treatments are not just scientific breakthroughs they represent hope for a more manageable future for those living with Fabry disease. With options like one-time gene therapy, oral alternatives, and biosimilar access, patients may soon enjoy longer lives with fewer medical burdens.
Although many of these therapies are still in clinical trials, the momentum is real. The coming years could bring a fundamental shift in how Fabry disease is diagnosed, treated, and lived with.

